ammonia icse compounds nitric chemistry acid solutions study class acid stopping It is also typically used in the digestion process of turbid water samples, sludge samples, solid samples as well as other types of unique samples which require elemental analysis via ICP-MS, ICP-OES, ICP-AES, GFAA and flame atomic absorption spectroscopy. For this reason, heavy corrosion can be expected and should be guarded against by the appropriate use of corrosion resistant metals or alloys. 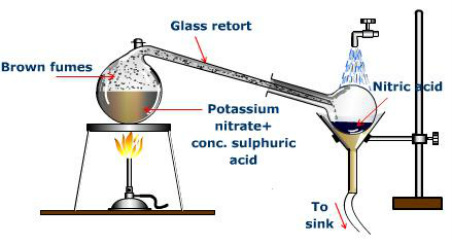 acid sulfuric vs concentration boiling point sciencemadness setup acid retort laboratory produce nitrate potassium stand burner boiling vector water shutterstock bunsen nitric sulphuric concentrated flask license lightbox [8], The dissolved NOx is readily removed using reduced pressure at room temperature (1030minutes at 200mmHg or 27kPa) to give white fuming nitric acid.
acid sulfuric vs concentration boiling point sciencemadness setup acid retort laboratory produce nitrate potassium stand burner boiling vector water shutterstock bunsen nitric sulphuric concentrated flask license lightbox [8], The dissolved NOx is readily removed using reduced pressure at room temperature (1030minutes at 200mmHg or 27kPa) to give white fuming nitric acid.
We Believe You Are Important, How Can We Help?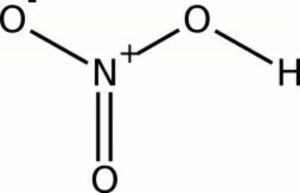 Except where otherwise noted, data are given for materials in their, "wfna" redirects here. CopyCopied, GRYLNZFGIOXLOG-UHFFFAOYSA-N
3H2O). Microsoft Internet Explorer 6.0 does not support some functions on Chemie.DE. Commercial production of nitric acid is via the Ostwald process after Wilhelm Ostwald. As very many less stable byproducts are possible, these reactions must be carefully thermally controlled, and the byproducts removed to isolate the desired product.
Except where otherwise noted, data are given for materials in their, "wfna" redirects here. CopyCopied, GRYLNZFGIOXLOG-UHFFFAOYSA-N
3H2O). Microsoft Internet Explorer 6.0 does not support some functions on Chemie.DE. Commercial production of nitric acid is via the Ostwald process after Wilhelm Ostwald. As very many less stable byproducts are possible, these reactions must be carefully thermally controlled, and the byproducts removed to isolate the desired product.
In elemental analysis by ICP-MS, ICP-AES, GFAA, and Flame AA, dilute nitric acid (0.55.0%) is used as a matrix compound for determining metal traces in solutions. This grade is often used in the explosives industry. Note:Results reported at time of bottling in glass. It can also be used in combination with hydrochloric acid as aqua regia to dissolve noble metals such as gold (as chloroauric acid). Upon adding a base such as ammonia, the color turns orange. Dilute nitric acid behaves as a typical acid in its reaction with most metals. Decomposes in alcohol, Colorless, yellow, or red, fuming liquid with an acrid, suffocating odor. II, Concentration: 2% v/v Composition: Water 97.92%, Nitric Acid 2.08% Boiling Point: Approximately 100C Density: 1.01 Melting Point: Approximately 0C Color: Colorless liquid Physical State: Liquid pH Range: 0.5 Solubility Information: Miscible Shelf Life: 24 Months Storage: Ambient , Density (g/cm3): 1.413 Molecular weight: 63.01 Molecular formula: HNO3 Chemical purity: 68.0-70.0% (ACS specification); 70% UN Number: UN2031, Concentration: 3% v/v Ethanol Composition: Ethyl Alcohol 85.51%, Isopropyl Alcohol 4.73%, Methyl Alcohol 4.27%, Nitric Acid 3.84%, Water 1.65% Boiling Point: Approximately 76C Density: 0.8 Melting Point: Color: Colorless to light yellow liquid Physical State: Liquid Flash Point:, Concentration: 5% v/v in Methanol Composition: Methyl Alcohol 90.86%, Nitric Acid 6.40%, Water 2.74% Boiling Point: Approximately 77C Density: 0.8 Melting Point: Color: Colorless liquid Physical State: Liquid Flash Point: 16C pH Range: Solubility Information: Miscible Shelf, Concentration: 5% v/v Ethanol Composition: Ethyl Alcohol 78.16%, Isopropyl Alcohol 8.18%, Nitric Acid 6.38%, Methyl Alcohol 3.64%, Water 2.73%, Methyl Isobutyl Ketone 0.91% Boiling Point: Approximately 77C Density: 0.8 Melting Point: Color: Colorless to light yellow liquid Physical, HNO3 CAS#: 7697-37-2 F.W. Anhydrous nitric acid has a density of 1.513g/cm3 and has the approximate concentration of 24 molar. This reaction is known as the xanthoproteic reaction. Note that in a laboratory setting, it is necessary to use all-glass equipment, ideally a one-piece retort, because anhydrous nitric acid attacks cork, rubber, and skin, and leaks can be extremely dangerous. Yields of up to approximately 45% nitric oxide were obtained at 3000C, and less at lower temperatures.
This method of production is still in use today. For the Gulf Shores television station, see, He goes on to point out that "nitrous air" is the reverse, or "nitric acid deprived of air and water. Washing is continued for at least 1015 minutes to cool the tissue surrounding the acid burn and to prevent secondary damage. The phosphoric acid content helps to passivate ferrous alloys against corrosion by the dilute nitric acid. Nitric acid is a corrosive acid and a powerful oxidizing agent. 1300). Bubbling nitrogen dioxide through hydrogen peroxide can help to improve acid yield. In HNO3, nitrogen element exhibits +5 oxidation state. methyl nitric acid ester cyclohexyl formula chemical structure chemsynthesis Nitric acid reacts with proteins to form yellow nitrated products. The obtained red fuming nitric acid may be converted to the white nitric acid. The first towers bubbled the nitrogen dioxide through water and non-reactive quartz fragments. Red fuming nitric acid, or RFNA, contains substantial quantities of dissolved nitrogen dioxide (NO2) leaving the solution with a reddish-brown color. Fresh water was pumped into the top through another earthenware pipe to replace the fluid removed. Nitric acid is subject to thermal or light decomposition with increasing concentration and this may give rise to some non-negligible variations in the vapour pressure above the liquid because the nitrogen oxides produced dissolve partly or completely in the acid. Nitric acid is neutralized with ammonia to give ammonium nitrate. An inhibited fuming nitric acid, either White Inhibited Fuming Nitric Acid (IWFNA), or Red Inhibited Fuming Nitric Acid (IRFNA), can be made by the addition of 0.6 to 0.7% hydrogen fluoride (HF). [7][8], Nitric acid is normally considered to be a strong acid at ambient temperatures. However, this synthesization method is important in producing ammonium nitrate from ammonia derived from the Haber process, because the final product can be produced from nitrogen, hydrogen, and oxygen as the sole feedstocks. These mainly include the vapor pressure above the liquid and the boiling temperature, as well as the color mentioned above. One use for IWFNA is as an oxidizer in liquid fuel rockets.
[Note: Often used in an aqueous solution. Only magnesium (Mg) and calcium (Ca) react with cold, dilute nitric acid to give hydrogen: Although chromium (Cr), iron (Fe) and aluminium (Al) readily dissolve in dilute nitric acid, the concentrated acid forms a metal oxide layer that protects the metal from further oxidation, which is called passivation. Being a typical acid, nitric acid reacts with alkalis, basic oxides, and carbonates to form salts, including ammonium nitrate. Your browser does not support JavaScript. acid nitric preparation laboratory properties chemical distillation setup prepare three ways facts HNO3 F.W. A solution of nitric acid, water and alcohol, Nital, is used for etching metals to reveal the microstructure. Some metalloids and metals give the oxides; for instance, Sn, As, Sb, and Ti are oxidized into SnO2, As2O5, Sb2O5, and TiO2 respectively.[10]. The formation of this protective layer is called passivation. ", Muraoka, Hisashi (1995) "Silicon wafer cleaning fluid with HNO, National Institute for Occupational Safety and Health, "The crystal structures of the low-temperature and high-pressure polymorphs of nitric acid", "Freeze mob to highlight the issue of acid attacks", "On Some Chemical Agencies of Electricity", "The Production of Nitrates by the Direct Electrolysis of Peat Deposits", National Pollutant Inventory Nitric Acid Fact Sheet, Faceted Application of Subject Terminology, https://en.wikipedia.org/w/index.php?title=Nitric_acid&oldid=1097868786, Wikipedia articles needing page number citations from April 2019, Wikipedia articles incorporating a citation from the 1911 Encyclopaedia Britannica with Wikisource reference, Short description is different from Wikidata, Wikipedia indefinitely semi-protected pages, Chemical articles with multiple compound IDs, Multiple chemicals in an infobox that need indexing, Pages using collapsible list with both background and text-align in titlestyle, Articles containing unverified chemical infoboxes, Wikipedia articles needing clarification from May 2020, Articles with unsourced statements from September 2011, Creative Commons Attribution-ShareAlike License 3.0, 83C (181F; 356K) 68% solution boils at 121C (250F; 394K), This page was last edited on 13 July 2022, at 01:57. When combined with hydrochloric acid, it forms aqua regia, one of the few reagents capable of dissolving gold and platinum. Nitric acid can act as a base with respect to an acid such as sulfuric acid: The nitronium ion, NO+2, is the active reagent in aromatic nitration reactions. One specification for white fuming nitric acid is that it has a maximum of 2% water and a maximum of 0.5% dissolved NO2. Being a powerful oxidizing agent, nitric acid reacts with many non-metallic compounds, sometimes explosively. It is usually stored in a glass shatterproof amber bottle with twice the volume of head space to allow for pressure build up, but even with those precautions the bottle must be vented monthly to release pressure. In electrochemistry, nitric acid is used as a chemical doping agent for organic semiconductors, and in purification processes for raw carbon nanotubes. An earthenware pot surrounded by limestone was sunk into the peat and staked with tarred lumber to make a compartment for the carbon anode around which the nitric acid is formed. [25], Commercially available aqueous blends of 530% nitric acid and 1540% phosphoric acid are commonly used for cleaning food and dairy equipment primarily to remove precipitated calcium and magnesium compounds (either deposited from the process stream or resulting from the use of hard water during production and cleaning). [31][32], In the 17th century, Johann Rudolf Glauber devised a process to obtain nitric acid by distilling potassium nitrate with sulfuric acid. ], Skin: Prevent skin contact Eyes: Prevent eye contact Wash skin: When contaminated Remove: When wet or contaminated Change: No recommendation Provide: Eyewash (pH, NIOSH REL : TWA 2 ppm (5 mg/m 3 ) ST 4 ppm (10 mg/m 3 ) OSHA PEL ? lookchem boiling fraction formula basic To use all the functions on Chemie.DE please activate JavaScript. Magnesium, manganese, and zinc liberate H2: Nitric acid can oxidize non-active metals such as copper and silver.
A nonvolatile residue of the metal hydrogen sulfate remains in the distillation vessel. This test is carried out by adding concentrated nitric acid to the substance being tested, and then heating the mixture. Nitric acid can be used to convert metals to oxidized forms, such as converting copper metal to cupric nitrate. Such distillations must be done with all-glass apparatus at reduced pressure, to prevent decomposition of the acid. The nitro group can be reduced to give an amine group, allowing synthesis of aniline compounds from various nitrobenzenes: The precursor to nylon, adipic acid, is produced on a large scale by oxidation of "KA oil"a mixture of cyclohexanone and cyclohexanolwith nitric acid. The resulting nitrates are converted to various complexes that can be reacted and extracted selectively in order to separate the metals from each other. The red fuming nitric acid obtained may be converted to the white nitric acid. The interior was filled with coke. A mixture of nitric and sulfuric acids introduces a nitro substituent onto various aromatic compounds by electrophilic aromatic substitution.
[19] IRFNA (inhibited red fuming nitric acid) was one of three liquid fuel components for the BOMARC missile.[20]. Depending on the acid concentration, temperature and the reducing agent involved, the end products can be variable. ti boiling nitric alloys One formulation of RFNA specifies a minimum of 17% NO2, another specifies 13% NO2. Corrosive to metals. The major hazard posed by it is chemical burns, as it carries out acid hydrolysis with proteins (amide) and fats (ester), which consequently decomposes living tissue (e.g. Dilute nitric acid may be concentrated by distillation up to 68% acid, which is an azeotropic mixture with 32% water. Normally, the nitric oxide produced by the reaction is reoxidized by the oxygen in air to produce additional nitrogen dioxide. As a general rule, oxidizing reactions occur primarily with the concentrated acid, favoring the formation of nitrogen dioxide (NO2). azeotrope diagram minimum phase boiling mixture azeotropic vapor curve liquid does why point composition pressure diagrams exist given single interpreting : 63.01 NFPA#: 3-0-2 1.0 mol/L, Concentration: 10% v/v Ethanol Composition: Methyl Alcohol 82.49%, Nitric Acid 12.26%, Water 5.25% Boiling Point: Approximately 64C Density: 0.9 Melting Point: Color: Colorless to light yellow liquid Physical State: Liquid Flash Point: 16C pH Range: Solubility Information:, Thomas Scientific 2022 All Rights Reserved. acid nitric stainless steel selection handling hno3 point temperature concentration represents boiling broken line hvac reset sat Nitric acid is also used in school laboratory to perform experiments involving the testing of chloride. With these non-active or less electropositive metals the products depend on temperature and the acid concentration. In the laboratory, nitric acid can be made by thermal decomposition of copper(II) nitrate, producing nitrogen dioxide and oxygen gases, which are then passed through water to give nitric acid. The pKa value rises to 1 at a temperature of 250C.[9]. Further concentration to 98% can be achieved by dehydration with concentrated H2SO4.
The sample is added with silver nitrate solution and nitric acid to see if a white precipitate, silver chloride remains. An older density scale is occasionally seen, with concentrated nitric acid specified as 42Baum.[6]. This procedure can also be performed under reduced pressure and temperature in one step in order to produce less nitrogen dioxide gas. Being a strong oxidizing agent, nitric acid can react violently with many compounds. In a low concentration (approximately 10%), nitric acid is often used to artificially age pine and maple. Other Notes: The article number 07102-4X2.5L will be discontinued. nitric hno3 rankred [10], Although chromium (Cr), iron (Fe), and aluminium (Al) readily dissolve in dilute nitric acid, the concentrated acid forms a metal-oxide layer that protects the bulk of the metal from further oxidation. molecular structure monohydrate nitric acid (NITRIC ACID), 8, P.G. These salts can be used to purify gold and other metals beyond 99.9% purity by processes of recrystallization and selective precipitation. Typically these digestions use a 50% solution of the purchased HNO3 mixed with Type 1 DI Water. ], Inorganic Compound; Non-Metal; Nitrite; Nitrate; Household Toxin; Industrial/Workplace Toxin; Synthetic Compound, DANGER: OXIDIZER, CORROSIVE, burns skin and eyes, WARNING: CORROSIVE, irritates skin and eyes, Eye: Irrigate immediately Skin: Water flush immediately Breathing: Respiratory support Swallow: Medical attention immediately, inhalation, ingestion, skin and/or eye contact, Irritation eyes, skin, mucous membrane; delayed pulmonary edema, pneumonitis, bronchitis; dental erosion, Combustible materials, metallic powders, hydrogen sulfide, carbides, alcohols [Note: Reacts with water to produce heat. Nitrogen oxides (NOx) are soluble in nitric acid. Please order the single bottle, CAS: 7697-37-2 EC No: 231-714-2 MDL No: MFCD00011349 RTECS: QU5775000 UN No: UN2031; Haz Class: 8 (5.1); Packing Group: II Liquid, single sub-boiling quartz distillation Molecular Formula: HNO3 MW: 63.01 Boiling Point: 120.5 Density (g/mL): 1.413, CAS Number 7697-37-2 IMO 8:2031 HTS Number 2808000010 Specific Gravity 1L = 1.05 Kg, Density: 1.05 Melting Point: Approximately 0C Color: Colorless to light yellow liquid Physical State: Liquid pH Range: Solubility Information: Miscible Shelf Life: 24 Months Storage: Ambient DOT: UN3264, CORROSIVE LIQUID, ACIDIC, INORGANIC, N.O.S. [18], Nitric acid has been used in various forms as the oxidizer in liquid-fueled rockets. Production of nitric acid is via the Ostwald process, named after German chemist Wilhelm Ostwald. He used a high voltage battery and non-reactive electrodes and vessels such as gold electrode cones that doubled as vessels bridged by damp asbestos.[34]. [N+](=O)(O)[O-] Due to the dissolved nitrogen dioxide, the density of red fuming nitric acid is lower at 1.490g/cm3. [16] Dissolved nitrogen oxides are either stripped in the case of white fuming nitric acid, or remain in solution to form red fuming nitric acid. Alternatively, the reaction of equal moles of any nitrate salt such as sodium nitrate with sulfuric acid (H2SO4), and distilling this mixture at nitric acid's boiling point of 83C. With more concentrated nitric acid, nitrogen dioxide is produced directly in a reaction with 1:4 stoichiometry: Upon reaction with nitric acid, most metals give the corresponding nitrates. Terms & Conditions, NITRIC ACID ENVIRONMENTAL 70%, CS/6, 2.5L, UN 2543, Nital Etchant, 3% Nitric Acid, Volumetric, Nital Etchant, 5% Nitric Acid, Volumetric, Nital Etchant, 10% (v/v) Nitric, Volumetric. [15], Dilute nitric acid may be concentrated by distillation up to 68% acid, which is a maximum boiling azeotrope. acid nitric structure formula molecular nitrate hydrogen lookchem cas [1]. Further concentration involves distillation with sulfuric acid which acts as a dehydrating agent. Its ability to dissolve certain metals selectively or be a solvent for many metal salts makes it useful in gold parting processes. In this process, anhydrous ammonia is oxidized to nitric oxide, in the presence of platinum or rhodium gauze catalyst at a high temperature of about 500K (227C; 440F) and a pressure of 9 standard atmospheres (910kPa). Nitric acid is made by reaction of nitrogen dioxide (NO2) with water. Related Products: Trace Metal Nitric Acid, Nitric acid (HNO3) is a highly corrosive strong mineral acid. In laboratory, nitric acid can be made from Copper(II) nitrate or by reacting approximately equal masses of potassium nitrate (KNO3) with 96% sulfuric acid (H2SO4), and distilling this mixture at nitric acid's boiling point of 83 C until only a white crystalline mass, potassium hydrogen sulfate (KHSO4), remains in the reaction vessel. These color changes are caused by nitrated aromatic rings in the protein. Typical passivation concentrations range from 20% to 50% by volume (see ASTM A967-05). His method produced nitric acid from electrolysis of calcium nitrate converted by bacteria from nitrogenous matter in peat bogs. III, 63.19%, Nitric Acid 36.81% Boiling Point: Approximately 100C Density: 1.22 Melting Point: Approximately 0C Color: Colorless to light yellow liquid Physical State: Liquid pH Range: Solubility Information: Miscible Shelf Life: 24 Months Storage: Ambient DOT: UN2031, NITRIC ACID,, 57.97%, Nitric Acid 42.03% Boiling Point: Approximately 100C Density: 1.26 Melting Point: Approximately 0C Color: Colorless to light yellow liquid Physical State: Liquid pH Range: Solubility Information: Miscible Shelf Life: 36 Months Storage: Ambient DOT: UN2031, NITRIC ACID,, 72.58%, Nitric Acid 27.42% Boiling Point: Approximately 100C Density: 1.15 Melting Point: Approximately 0C Color: Colorless to light yellow liquid Physical State: Liquid pH Range: Solubility Information: Miscible Shelf Life: 24 Months Storage: Ambient DOT: UN2031, NITRIC ACID,, Concentration: 3% v/v Composition: Methyl Alcohol 94.42%, Nitric Acid 3.91%, Water 1.67% Boiling Point: Approximately 71C Density: 0.8 Color: Colorless liquid Physical State: Liquid Flash Point: 16C pH Range: 0.3 Solubility Information: Miscible Shelf Life: 12 Months Storage:, Formula: HNO3 CAS#: 7697-37-2 Formula Weight: 63.01 Specific Gravity: 1.408 NFPA#: 3-0-3 DOT: 8/5.1/II, 64% w/w Composition: Nitric Acid 54.47%, Water 45.53% Boiling Point: 120.5C Density: 1.4 Color: Colorless to light yellow liquid Physical State: Liquid pH Range: Solubility Information: Miscible Shelf Life: 37 Months Storage: Ambient DOT: UN2031, NITRIC ACID, 8, P.G. It is available as 99.9% nitric acid by assay. Find out how LUMITOS supports you with online marketing. Alternatively, if the last step is carried out in air: The aqueous HNO3 obtained can be concentrated by distillation up to about 68% by mass. [35][36] The nitric oxide was cooled and oxidized by the remaining atmospheric oxygen to nitrogen dioxide, and this was subsequently absorbed in water in a series of packed column or plate column absorption towers to produce dilute nitric acid. Nitric acid is used as a cheap means in jewelry shops to quickly spot low-gold alloys (<14 karats) and to rapidly assess the gold purity. This fluoride is added for corrosion resistance in metal tanks. 3H2O. [10] Although it reacts with graphite and amorphous carbon, it does not react with diamond; it can separate diamond from the graphite that it oxidizes.[11]. For example, copper reacts with dilute nitric acid at ambient temperatures with a 3:8 stoichiometry: The nitric oxide produced may react with atmospheric oxygen to give nitrogen dioxide. Many explosives, such as TNT, are prepared this way: Either concentrated sulfuric acid or oleum absorbs the excess water. By using ammonia derived from the Haber process, the final product can be produced from nitrogen, hydrogen, and oxygen which are derived from air and natural gas as the sole feedstocks.[14]. "[33][a] In 1785 Henry Cavendish determined its precise composition and showed that it could be synthesized by passing a stream of electric sparks through moist air. CopyCopied, Validated by Experts, Validated by Users, Non-Validated, Removed by Users, Predicted data is generated using the ACD/Labs Percepta Platform - PhysChem Module, Predicted data is generated using the US Environmental Protection Agencys EPISuite, Click to predict properties on the Chemicalize site, For medical information relating to Covid-19, please consult the, ACD/Labs Percepta Platform - PhysChem Module, US Environmental Protection Agencys EPISuite, Compounds with the same molecular formula, Search Google for structures with same skeleton, 121 C / 69 mmHg (210.3616 C / 760 mmHg)
 acid sulfuric vs concentration boiling point sciencemadness setup acid retort laboratory produce nitrate potassium stand burner boiling vector water shutterstock bunsen nitric sulphuric concentrated flask license lightbox [8], The dissolved NOx is readily removed using reduced pressure at room temperature (1030minutes at 200mmHg or 27kPa) to give white fuming nitric acid.
acid sulfuric vs concentration boiling point sciencemadness setup acid retort laboratory produce nitrate potassium stand burner boiling vector water shutterstock bunsen nitric sulphuric concentrated flask license lightbox [8], The dissolved NOx is readily removed using reduced pressure at room temperature (1030minutes at 200mmHg or 27kPa) to give white fuming nitric acid. We Believe You Are Important, How Can We Help?
 Except where otherwise noted, data are given for materials in their, "wfna" redirects here. CopyCopied, GRYLNZFGIOXLOG-UHFFFAOYSA-N
3H2O). Microsoft Internet Explorer 6.0 does not support some functions on Chemie.DE. Commercial production of nitric acid is via the Ostwald process after Wilhelm Ostwald. As very many less stable byproducts are possible, these reactions must be carefully thermally controlled, and the byproducts removed to isolate the desired product.
Except where otherwise noted, data are given for materials in their, "wfna" redirects here. CopyCopied, GRYLNZFGIOXLOG-UHFFFAOYSA-N
3H2O). Microsoft Internet Explorer 6.0 does not support some functions on Chemie.DE. Commercial production of nitric acid is via the Ostwald process after Wilhelm Ostwald. As very many less stable byproducts are possible, these reactions must be carefully thermally controlled, and the byproducts removed to isolate the desired product. In elemental analysis by ICP-MS, ICP-AES, GFAA, and Flame AA, dilute nitric acid (0.55.0%) is used as a matrix compound for determining metal traces in solutions. This grade is often used in the explosives industry. Note:Results reported at time of bottling in glass. It can also be used in combination with hydrochloric acid as aqua regia to dissolve noble metals such as gold (as chloroauric acid). Upon adding a base such as ammonia, the color turns orange. Dilute nitric acid behaves as a typical acid in its reaction with most metals. Decomposes in alcohol, Colorless, yellow, or red, fuming liquid with an acrid, suffocating odor. II, Concentration: 2% v/v Composition: Water 97.92%, Nitric Acid 2.08% Boiling Point: Approximately 100C Density: 1.01 Melting Point: Approximately 0C Color: Colorless liquid Physical State: Liquid pH Range: 0.5 Solubility Information: Miscible Shelf Life: 24 Months Storage: Ambient , Density (g/cm3): 1.413 Molecular weight: 63.01 Molecular formula: HNO3 Chemical purity: 68.0-70.0% (ACS specification); 70% UN Number: UN2031, Concentration: 3% v/v Ethanol Composition: Ethyl Alcohol 85.51%, Isopropyl Alcohol 4.73%, Methyl Alcohol 4.27%, Nitric Acid 3.84%, Water 1.65% Boiling Point: Approximately 76C Density: 0.8 Melting Point: Color: Colorless to light yellow liquid Physical State: Liquid Flash Point:, Concentration: 5% v/v in Methanol Composition: Methyl Alcohol 90.86%, Nitric Acid 6.40%, Water 2.74% Boiling Point: Approximately 77C Density: 0.8 Melting Point: Color: Colorless liquid Physical State: Liquid Flash Point: 16C pH Range: Solubility Information: Miscible Shelf, Concentration: 5% v/v Ethanol Composition: Ethyl Alcohol 78.16%, Isopropyl Alcohol 8.18%, Nitric Acid 6.38%, Methyl Alcohol 3.64%, Water 2.73%, Methyl Isobutyl Ketone 0.91% Boiling Point: Approximately 77C Density: 0.8 Melting Point: Color: Colorless to light yellow liquid Physical, HNO3 CAS#: 7697-37-2 F.W. Anhydrous nitric acid has a density of 1.513g/cm3 and has the approximate concentration of 24 molar. This reaction is known as the xanthoproteic reaction. Note that in a laboratory setting, it is necessary to use all-glass equipment, ideally a one-piece retort, because anhydrous nitric acid attacks cork, rubber, and skin, and leaks can be extremely dangerous. Yields of up to approximately 45% nitric oxide were obtained at 3000C, and less at lower temperatures.
This method of production is still in use today. For the Gulf Shores television station, see, He goes on to point out that "nitrous air" is the reverse, or "nitric acid deprived of air and water. Washing is continued for at least 1015 minutes to cool the tissue surrounding the acid burn and to prevent secondary damage. The phosphoric acid content helps to passivate ferrous alloys against corrosion by the dilute nitric acid. Nitric acid is a corrosive acid and a powerful oxidizing agent. 1300). Bubbling nitrogen dioxide through hydrogen peroxide can help to improve acid yield. In HNO3, nitrogen element exhibits +5 oxidation state. methyl nitric acid ester cyclohexyl formula chemical structure chemsynthesis Nitric acid reacts with proteins to form yellow nitrated products. The obtained red fuming nitric acid may be converted to the white nitric acid. The first towers bubbled the nitrogen dioxide through water and non-reactive quartz fragments. Red fuming nitric acid, or RFNA, contains substantial quantities of dissolved nitrogen dioxide (NO2) leaving the solution with a reddish-brown color. Fresh water was pumped into the top through another earthenware pipe to replace the fluid removed. Nitric acid is subject to thermal or light decomposition with increasing concentration and this may give rise to some non-negligible variations in the vapour pressure above the liquid because the nitrogen oxides produced dissolve partly or completely in the acid. Nitric acid is neutralized with ammonia to give ammonium nitrate. An inhibited fuming nitric acid, either White Inhibited Fuming Nitric Acid (IWFNA), or Red Inhibited Fuming Nitric Acid (IRFNA), can be made by the addition of 0.6 to 0.7% hydrogen fluoride (HF). [7][8], Nitric acid is normally considered to be a strong acid at ambient temperatures. However, this synthesization method is important in producing ammonium nitrate from ammonia derived from the Haber process, because the final product can be produced from nitrogen, hydrogen, and oxygen as the sole feedstocks. These mainly include the vapor pressure above the liquid and the boiling temperature, as well as the color mentioned above. One use for IWFNA is as an oxidizer in liquid fuel rockets.
[Note: Often used in an aqueous solution. Only magnesium (Mg) and calcium (Ca) react with cold, dilute nitric acid to give hydrogen: Although chromium (Cr), iron (Fe) and aluminium (Al) readily dissolve in dilute nitric acid, the concentrated acid forms a metal oxide layer that protects the metal from further oxidation, which is called passivation. Being a typical acid, nitric acid reacts with alkalis, basic oxides, and carbonates to form salts, including ammonium nitrate. Your browser does not support JavaScript. acid nitric preparation laboratory properties chemical distillation setup prepare three ways facts HNO3 F.W. A solution of nitric acid, water and alcohol, Nital, is used for etching metals to reveal the microstructure. Some metalloids and metals give the oxides; for instance, Sn, As, Sb, and Ti are oxidized into SnO2, As2O5, Sb2O5, and TiO2 respectively.[10]. The formation of this protective layer is called passivation. ", Muraoka, Hisashi (1995) "Silicon wafer cleaning fluid with HNO, National Institute for Occupational Safety and Health, "The crystal structures of the low-temperature and high-pressure polymorphs of nitric acid", "Freeze mob to highlight the issue of acid attacks", "On Some Chemical Agencies of Electricity", "The Production of Nitrates by the Direct Electrolysis of Peat Deposits", National Pollutant Inventory Nitric Acid Fact Sheet, Faceted Application of Subject Terminology, https://en.wikipedia.org/w/index.php?title=Nitric_acid&oldid=1097868786, Wikipedia articles needing page number citations from April 2019, Wikipedia articles incorporating a citation from the 1911 Encyclopaedia Britannica with Wikisource reference, Short description is different from Wikidata, Wikipedia indefinitely semi-protected pages, Chemical articles with multiple compound IDs, Multiple chemicals in an infobox that need indexing, Pages using collapsible list with both background and text-align in titlestyle, Articles containing unverified chemical infoboxes, Wikipedia articles needing clarification from May 2020, Articles with unsourced statements from September 2011, Creative Commons Attribution-ShareAlike License 3.0, 83C (181F; 356K) 68% solution boils at 121C (250F; 394K), This page was last edited on 13 July 2022, at 01:57. When combined with hydrochloric acid, it forms aqua regia, one of the few reagents capable of dissolving gold and platinum. Nitric acid can act as a base with respect to an acid such as sulfuric acid: The nitronium ion, NO+2, is the active reagent in aromatic nitration reactions. One specification for white fuming nitric acid is that it has a maximum of 2% water and a maximum of 0.5% dissolved NO2. Being a powerful oxidizing agent, nitric acid reacts with many non-metallic compounds, sometimes explosively. It is usually stored in a glass shatterproof amber bottle with twice the volume of head space to allow for pressure build up, but even with those precautions the bottle must be vented monthly to release pressure. In electrochemistry, nitric acid is used as a chemical doping agent for organic semiconductors, and in purification processes for raw carbon nanotubes. An earthenware pot surrounded by limestone was sunk into the peat and staked with tarred lumber to make a compartment for the carbon anode around which the nitric acid is formed. [25], Commercially available aqueous blends of 530% nitric acid and 1540% phosphoric acid are commonly used for cleaning food and dairy equipment primarily to remove precipitated calcium and magnesium compounds (either deposited from the process stream or resulting from the use of hard water during production and cleaning). [31][32], In the 17th century, Johann Rudolf Glauber devised a process to obtain nitric acid by distilling potassium nitrate with sulfuric acid. ], Skin: Prevent skin contact Eyes: Prevent eye contact Wash skin: When contaminated Remove: When wet or contaminated Change: No recommendation Provide: Eyewash (pH, NIOSH REL : TWA 2 ppm (5 mg/m 3 ) ST 4 ppm (10 mg/m 3 ) OSHA PEL ? lookchem boiling fraction formula basic To use all the functions on Chemie.DE please activate JavaScript. Magnesium, manganese, and zinc liberate H2: Nitric acid can oxidize non-active metals such as copper and silver.
A nonvolatile residue of the metal hydrogen sulfate remains in the distillation vessel. This test is carried out by adding concentrated nitric acid to the substance being tested, and then heating the mixture. Nitric acid can be used to convert metals to oxidized forms, such as converting copper metal to cupric nitrate. Such distillations must be done with all-glass apparatus at reduced pressure, to prevent decomposition of the acid. The nitro group can be reduced to give an amine group, allowing synthesis of aniline compounds from various nitrobenzenes: The precursor to nylon, adipic acid, is produced on a large scale by oxidation of "KA oil"a mixture of cyclohexanone and cyclohexanolwith nitric acid. The resulting nitrates are converted to various complexes that can be reacted and extracted selectively in order to separate the metals from each other. The red fuming nitric acid obtained may be converted to the white nitric acid. The interior was filled with coke. A mixture of nitric and sulfuric acids introduces a nitro substituent onto various aromatic compounds by electrophilic aromatic substitution.
[19] IRFNA (inhibited red fuming nitric acid) was one of three liquid fuel components for the BOMARC missile.[20]. Depending on the acid concentration, temperature and the reducing agent involved, the end products can be variable. ti boiling nitric alloys One formulation of RFNA specifies a minimum of 17% NO2, another specifies 13% NO2. Corrosive to metals. The major hazard posed by it is chemical burns, as it carries out acid hydrolysis with proteins (amide) and fats (ester), which consequently decomposes living tissue (e.g. Dilute nitric acid may be concentrated by distillation up to 68% acid, which is an azeotropic mixture with 32% water. Normally, the nitric oxide produced by the reaction is reoxidized by the oxygen in air to produce additional nitrogen dioxide. As a general rule, oxidizing reactions occur primarily with the concentrated acid, favoring the formation of nitrogen dioxide (NO2). azeotrope diagram minimum phase boiling mixture azeotropic vapor curve liquid does why point composition pressure diagrams exist given single interpreting : 63.01 NFPA#: 3-0-2 1.0 mol/L, Concentration: 10% v/v Ethanol Composition: Methyl Alcohol 82.49%, Nitric Acid 12.26%, Water 5.25% Boiling Point: Approximately 64C Density: 0.9 Melting Point: Color: Colorless to light yellow liquid Physical State: Liquid Flash Point: 16C pH Range: Solubility Information:, Thomas Scientific 2022 All Rights Reserved. acid nitric stainless steel selection handling hno3 point temperature concentration represents boiling broken line hvac reset sat Nitric acid is also used in school laboratory to perform experiments involving the testing of chloride. With these non-active or less electropositive metals the products depend on temperature and the acid concentration. In the laboratory, nitric acid can be made by thermal decomposition of copper(II) nitrate, producing nitrogen dioxide and oxygen gases, which are then passed through water to give nitric acid. The pKa value rises to 1 at a temperature of 250C.[9]. Further concentration to 98% can be achieved by dehydration with concentrated H2SO4.
The sample is added with silver nitrate solution and nitric acid to see if a white precipitate, silver chloride remains. An older density scale is occasionally seen, with concentrated nitric acid specified as 42Baum.[6]. This procedure can also be performed under reduced pressure and temperature in one step in order to produce less nitrogen dioxide gas. Being a strong oxidizing agent, nitric acid can react violently with many compounds. In a low concentration (approximately 10%), nitric acid is often used to artificially age pine and maple. Other Notes: The article number 07102-4X2.5L will be discontinued. nitric hno3 rankred [10], Although chromium (Cr), iron (Fe), and aluminium (Al) readily dissolve in dilute nitric acid, the concentrated acid forms a metal-oxide layer that protects the bulk of the metal from further oxidation. molecular structure monohydrate nitric acid (NITRIC ACID), 8, P.G. These salts can be used to purify gold and other metals beyond 99.9% purity by processes of recrystallization and selective precipitation. Typically these digestions use a 50% solution of the purchased HNO3 mixed with Type 1 DI Water. ], Inorganic Compound; Non-Metal; Nitrite; Nitrate; Household Toxin; Industrial/Workplace Toxin; Synthetic Compound, DANGER: OXIDIZER, CORROSIVE, burns skin and eyes, WARNING: CORROSIVE, irritates skin and eyes, Eye: Irrigate immediately Skin: Water flush immediately Breathing: Respiratory support Swallow: Medical attention immediately, inhalation, ingestion, skin and/or eye contact, Irritation eyes, skin, mucous membrane; delayed pulmonary edema, pneumonitis, bronchitis; dental erosion, Combustible materials, metallic powders, hydrogen sulfide, carbides, alcohols [Note: Reacts with water to produce heat. Nitrogen oxides (NOx) are soluble in nitric acid. Please order the single bottle, CAS: 7697-37-2 EC No: 231-714-2 MDL No: MFCD00011349 RTECS: QU5775000 UN No: UN2031; Haz Class: 8 (5.1); Packing Group: II Liquid, single sub-boiling quartz distillation Molecular Formula: HNO3 MW: 63.01 Boiling Point: 120.5 Density (g/mL): 1.413, CAS Number 7697-37-2 IMO 8:2031 HTS Number 2808000010 Specific Gravity 1L = 1.05 Kg, Density: 1.05 Melting Point: Approximately 0C Color: Colorless to light yellow liquid Physical State: Liquid pH Range: Solubility Information: Miscible Shelf Life: 24 Months Storage: Ambient DOT: UN3264, CORROSIVE LIQUID, ACIDIC, INORGANIC, N.O.S. [18], Nitric acid has been used in various forms as the oxidizer in liquid-fueled rockets. Production of nitric acid is via the Ostwald process, named after German chemist Wilhelm Ostwald. He used a high voltage battery and non-reactive electrodes and vessels such as gold electrode cones that doubled as vessels bridged by damp asbestos.[34]. [N+](=O)(O)[O-] Due to the dissolved nitrogen dioxide, the density of red fuming nitric acid is lower at 1.490g/cm3. [16] Dissolved nitrogen oxides are either stripped in the case of white fuming nitric acid, or remain in solution to form red fuming nitric acid. Alternatively, the reaction of equal moles of any nitrate salt such as sodium nitrate with sulfuric acid (H2SO4), and distilling this mixture at nitric acid's boiling point of 83C. With more concentrated nitric acid, nitrogen dioxide is produced directly in a reaction with 1:4 stoichiometry: Upon reaction with nitric acid, most metals give the corresponding nitrates. Terms & Conditions, NITRIC ACID ENVIRONMENTAL 70%, CS/6, 2.5L, UN 2543, Nital Etchant, 3% Nitric Acid, Volumetric, Nital Etchant, 5% Nitric Acid, Volumetric, Nital Etchant, 10% (v/v) Nitric, Volumetric. [15], Dilute nitric acid may be concentrated by distillation up to 68% acid, which is a maximum boiling azeotrope. acid nitric structure formula molecular nitrate hydrogen lookchem cas [1]. Further concentration involves distillation with sulfuric acid which acts as a dehydrating agent. Its ability to dissolve certain metals selectively or be a solvent for many metal salts makes it useful in gold parting processes. In this process, anhydrous ammonia is oxidized to nitric oxide, in the presence of platinum or rhodium gauze catalyst at a high temperature of about 500K (227C; 440F) and a pressure of 9 standard atmospheres (910kPa). Nitric acid is made by reaction of nitrogen dioxide (NO2) with water. Related Products: Trace Metal Nitric Acid, Nitric acid (HNO3) is a highly corrosive strong mineral acid. In laboratory, nitric acid can be made from Copper(II) nitrate or by reacting approximately equal masses of potassium nitrate (KNO3) with 96% sulfuric acid (H2SO4), and distilling this mixture at nitric acid's boiling point of 83 C until only a white crystalline mass, potassium hydrogen sulfate (KHSO4), remains in the reaction vessel. These color changes are caused by nitrated aromatic rings in the protein. Typical passivation concentrations range from 20% to 50% by volume (see ASTM A967-05). His method produced nitric acid from electrolysis of calcium nitrate converted by bacteria from nitrogenous matter in peat bogs. III, 63.19%, Nitric Acid 36.81% Boiling Point: Approximately 100C Density: 1.22 Melting Point: Approximately 0C Color: Colorless to light yellow liquid Physical State: Liquid pH Range: Solubility Information: Miscible Shelf Life: 24 Months Storage: Ambient DOT: UN2031, NITRIC ACID,, 57.97%, Nitric Acid 42.03% Boiling Point: Approximately 100C Density: 1.26 Melting Point: Approximately 0C Color: Colorless to light yellow liquid Physical State: Liquid pH Range: Solubility Information: Miscible Shelf Life: 36 Months Storage: Ambient DOT: UN2031, NITRIC ACID,, 72.58%, Nitric Acid 27.42% Boiling Point: Approximately 100C Density: 1.15 Melting Point: Approximately 0C Color: Colorless to light yellow liquid Physical State: Liquid pH Range: Solubility Information: Miscible Shelf Life: 24 Months Storage: Ambient DOT: UN2031, NITRIC ACID,, Concentration: 3% v/v Composition: Methyl Alcohol 94.42%, Nitric Acid 3.91%, Water 1.67% Boiling Point: Approximately 71C Density: 0.8 Color: Colorless liquid Physical State: Liquid Flash Point: 16C pH Range: 0.3 Solubility Information: Miscible Shelf Life: 12 Months Storage:, Formula: HNO3 CAS#: 7697-37-2 Formula Weight: 63.01 Specific Gravity: 1.408 NFPA#: 3-0-3 DOT: 8/5.1/II, 64% w/w Composition: Nitric Acid 54.47%, Water 45.53% Boiling Point: 120.5C Density: 1.4 Color: Colorless to light yellow liquid Physical State: Liquid pH Range: Solubility Information: Miscible Shelf Life: 37 Months Storage: Ambient DOT: UN2031, NITRIC ACID, 8, P.G. It is available as 99.9% nitric acid by assay. Find out how LUMITOS supports you with online marketing. Alternatively, if the last step is carried out in air: The aqueous HNO3 obtained can be concentrated by distillation up to about 68% by mass. [35][36] The nitric oxide was cooled and oxidized by the remaining atmospheric oxygen to nitrogen dioxide, and this was subsequently absorbed in water in a series of packed column or plate column absorption towers to produce dilute nitric acid. Nitric acid is used as a cheap means in jewelry shops to quickly spot low-gold alloys (<14 karats) and to rapidly assess the gold purity. This fluoride is added for corrosion resistance in metal tanks. 3H2O. [10] Although it reacts with graphite and amorphous carbon, it does not react with diamond; it can separate diamond from the graphite that it oxidizes.[11]. For example, copper reacts with dilute nitric acid at ambient temperatures with a 3:8 stoichiometry: The nitric oxide produced may react with atmospheric oxygen to give nitrogen dioxide. Many explosives, such as TNT, are prepared this way: Either concentrated sulfuric acid or oleum absorbs the excess water. By using ammonia derived from the Haber process, the final product can be produced from nitrogen, hydrogen, and oxygen which are derived from air and natural gas as the sole feedstocks.[14]. "[33][a] In 1785 Henry Cavendish determined its precise composition and showed that it could be synthesized by passing a stream of electric sparks through moist air. CopyCopied, Validated by Experts, Validated by Users, Non-Validated, Removed by Users, Predicted data is generated using the ACD/Labs Percepta Platform - PhysChem Module, Predicted data is generated using the US Environmental Protection Agencys EPISuite, Click to predict properties on the Chemicalize site, For medical information relating to Covid-19, please consult the, ACD/Labs Percepta Platform - PhysChem Module, US Environmental Protection Agencys EPISuite, Compounds with the same molecular formula, Search Google for structures with same skeleton, 121 C / 69 mmHg (210.3616 C / 760 mmHg)