 Both are white solids, but samples are often contaminated with iron(III) chloride, giving a yellow color. These types of reactions are the major use for aluminium chloride, for example, in the preparation of anthraquinone (used in the dyestuffs industry) from benzene and phosgene. The anhydrous material is important commercially. [19][20], Aluminium chloride combined with aluminium in the presence of an arene can be used to synthesize bis(arene) metal complexes, e.g. bis(benzene)chromium, from certain metal halides via the so-called Fischer-Hafner synthesis. Your physics assignments can be a real challenge, and the due date can be really close feel free to use our assistance and get the desired result.
Both are white solids, but samples are often contaminated with iron(III) chloride, giving a yellow color. These types of reactions are the major use for aluminium chloride, for example, in the preparation of anthraquinone (used in the dyestuffs industry) from benzene and phosgene. The anhydrous material is important commercially. [19][20], Aluminium chloride combined with aluminium in the presence of an arene can be used to synthesize bis(arene) metal complexes, e.g. bis(benzene)chromium, from certain metal halides via the so-called Fischer-Hafner synthesis. Your physics assignments can be a real challenge, and the due date can be really close feel free to use our assistance and get the desired result.  The melt conducts electricity poorly,[9] unlike more ionic halides such as sodium chloride. (Chapter 2$)$\begin{equation}\begi, Chlorine The electron configuration of a chlorine atomis $[\mathrm{Ne}] .
The melt conducts electricity poorly,[9] unlike more ionic halides such as sodium chloride. (Chapter 2$)$\begin{equation}\begi, Chlorine The electron configuration of a chlorine atomis $[\mathrm{Ne}] .
They consist of aluminium and chlorine atoms in a 1:3 ratio, and one form also contains six waters of hydration. You question, we only have the show.  And they were formed Alan Hydroxide. [16], Aluminium chloride finds a wide variety of other applications in organic chemistry. Soln- AlCl3 + 3NaOH ----> Al(OH)3 + 3NaCl 1mol. AlCl3 adopts three structures, depending on the temperature and the state (solid, liquid, gas).
And they were formed Alan Hydroxide. [16], Aluminium chloride finds a wide variety of other applications in organic chemistry. Soln- AlCl3 + 3NaOH ----> Al(OH)3 + 3NaCl 1mol. AlCl3 adopts three structures, depending on the temperature and the state (solid, liquid, gas).
So the mine also the car Ryan for the computer Ionic equation. Your comments have been successfully added. Hydrated aluminium trichloride is prepared by dissolving aluminium oxides in hydrochloric acid. The alkylation reaction is more widely used than the acylation reaction, although its practice is more technically demanding. In the US in 1993, approximately 21,000 tons were produced, not counting the amounts consumed in the production of aluminium.[7]. we're giving the washing between albumin core I solution as sodium hydroxide solution and the reformer l William hydroxide saw that. h\;@D7~lK#P"@? University of Maryland - University College. A general problem with the FriedelCrafts reaction is that the aluminium chloride catalyst sometimes is required in full stoichiometric quantities, because it complexes strongly with the products. Adam had rocks. And dangers are spectator and they don't do anything. The hexahydrate consists of octahedral [Al(H2O)6]3+ centers and chloride counterions. Al2Cl6 dimers are also found in the vapour phase. 1mol. However, they need to be checked by the moderator before being published.  Anhydrous aluminium chloride is a powerful Lewis acid, capable of forming Lewis acid-base adducts with even weak Lewis bases such as benzophenone and mesitylene. c{| L?m, .$S Z:l\l`6jdw%X_cDN}NK& geVE>!0~f|)lr:yd'|U=oc %DLG xgh.g&pu6`ZGY r4`t{KY.x}woK~2g{?nn@.dP9,q0q4pQ4Y],6!f2E13~r9{ It has a low melting and boiling point. [9] In the general FriedelCrafts reaction, an acyl chloride or alkyl halide reacts with an aromatic system as shown:[11]. e&gM!A@PWI(zg$gT"%f3(sg7ao"u[ }1ltF[m Hb\RLuSYAQSJ$PlNnO(
K,Q>?>3JO`[Sa? hV_o0*~'RU :i6M{ Z#QRa~s. 4`tb=sq2,@:x P
endstream
endobj
214 0 obj
<>stream
Anhydrous aluminium chloride is a powerful Lewis acid, capable of forming Lewis acid-base adducts with even weak Lewis bases such as benzophenone and mesitylene. c{| L?m, .$S Z:l\l`6jdw%X_cDN}NK& geVE>!0~f|)lr:yd'|U=oc %DLG xgh.g&pu6`ZGY r4`t{KY.x}woK~2g{?nn@.dP9,q0q4pQ4Y],6!f2E13~r9{ It has a low melting and boiling point. [9] In the general FriedelCrafts reaction, an acyl chloride or alkyl halide reacts with an aromatic system as shown:[11]. e&gM!A@PWI(zg$gT"%f3(sg7ao"u[ }1ltF[m Hb\RLuSYAQSJ$PlNnO(
K,Q>?>3JO`[Sa? hV_o0*~'RU :i6M{ Z#QRa~s. 4`tb=sq2,@:x P
endstream
endobj
214 0 obj
<>stream
Dichlorophenylphosphine is prepared by reaction of benzene and phosphorus trichloride catalyzed by aluminium chloride.[21]. ). 3eJK:wX w+^0L1s!@{,7+RZhol9?W#iDVI
Y &/V*)\rox-(opCE~ rB9
 So he's there that on any question, which is their show off a little giant iron with three hydro sign. So the Mayan sweetie Hi, Rosa in and that of your form aluminum hydroxide. 3mol Molar mass of NaCl = 58.5g/mol Given. ionic and net ionic equations for an aqueous solution of aluminum chloride and sodium hydroxide that are mixed. Relate lattice energy to ionic-bond strength. [7], Aluminium chloride can also be used to introduce aldehyde groups onto aromatic rings, for example via the Gattermann-Koch reaction which uses carbon monoxide, hydrogen chloride and a copper(I) chloride co-catalyst. And also some core i after death, we also have to balance the chemical reaction abuse on the right. A1vjp zN6p\W
pG@
So he's there that on any question, which is their show off a little giant iron with three hydro sign. So the Mayan sweetie Hi, Rosa in and that of your form aluminum hydroxide. 3mol Molar mass of NaCl = 58.5g/mol Given. ionic and net ionic equations for an aqueous solution of aluminum chloride and sodium hydroxide that are mixed. Relate lattice energy to ionic-bond strength. [7], Aluminium chloride can also be used to introduce aldehyde groups onto aromatic rings, for example via the Gattermann-Koch reaction which uses carbon monoxide, hydrogen chloride and a copper(I) chloride co-catalyst. And also some core i after death, we also have to balance the chemical reaction abuse on the right. A1vjp zN6p\W
pG@  So we will would be our l let me, um, and had Rosa in for this lion in Korea. Thank you for the assistance.
So we will would be our l let me, um, and had Rosa in for this lion in Korea. Thank you for the assistance.  x{ xT9}osg$22$7! : Die Umlagerung des Cyclohexans in Metyl-cyclopentan", "Synthesis of Trifluoromethyl Ketones from Carboxylic Acids: 4-(3,4-Dibromophenyl)-1,1,1-trifluoro-4-methylpentan-2-one", Index of Organic Synthesis procedures that utilize AlCl, Government of Canada Fact Sheets and Frequently Asked Questions: Aluminum Salts, https://en.wikipedia.org/w/index.php?title=Aluminium_chloride&oldid=1084378384, Chemical articles with multiple compound IDs, Multiple chemicals in an infobox that need indexing, Chemical articles with multiple CAS registry numbers, Pages using collapsible list with both background and text-align in titlestyle, Articles containing unverified chemical infoboxes, Creative Commons Attribution-ShareAlike License 3.0, 180C (356F; 453K) (anhydrous, sublimes), Soluble in hydrogen chloride, ethanol, chloroform, carbon tetrachloride, This page was last edited on 24 April 2022, at 03:38. >jk
x{ xT9}osg$22$7! : Die Umlagerung des Cyclohexans in Metyl-cyclopentan", "Synthesis of Trifluoromethyl Ketones from Carboxylic Acids: 4-(3,4-Dibromophenyl)-1,1,1-trifluoro-4-methylpentan-2-one", Index of Organic Synthesis procedures that utilize AlCl, Government of Canada Fact Sheets and Frequently Asked Questions: Aluminum Salts, https://en.wikipedia.org/w/index.php?title=Aluminium_chloride&oldid=1084378384, Chemical articles with multiple compound IDs, Multiple chemicals in an infobox that need indexing, Chemical articles with multiple CAS registry numbers, Pages using collapsible list with both background and text-align in titlestyle, Articles containing unverified chemical infoboxes, Creative Commons Attribution-ShareAlike License 3.0, 180C (356F; 453K) (anhydrous, sublimes), Soluble in hydrogen chloride, ethanol, chloroform, carbon tetrachloride, This page was last edited on 24 April 2022, at 03:38. >jk  }:v/+|C ftukaQonD`
! %PDF-1.6
%
4-g@ d
MI&S20Rk`S6xpk
)( ;@ u@2)1, p=jf!AuJ{oGer3~Fu#pD%7_]hR?qln-[sczLJ:l]AZ@gUZOZ[gl2y j0x(^u-Ak][]s ' Zk! $l$T4QOt"y\b)AI&NI$R$)TIj"]&=&!:dGrY@^O$ _%?P(&OJEBN9J@y@yCR
nXZOD}J}/G3k{%Ow_.'_!JQ@SVF=IEbbbb5Q%O@%!ByM:e0G7 e%e[(R0`3R46i^)*n*|"fLUomO0j&jajj.w_4zj=U45n4hZZZ^0Tf%9->=cXgN]. [23][22], Anhydrous AlCl3 reacts vigorously with bases, so suitable precautions are required. For these and similar reasons, the use of aluminium chloride has often been displaced by zeolites. ^{%\RoXk
>Q+k;EOA1^6ziN
(UBn9(yLAz?!B8J`t
}:v/+|C ftukaQonD`
! %PDF-1.6
%
4-g@ d
MI&S20Rk`S6xpk
)( ;@ u@2)1, p=jf!AuJ{oGer3~Fu#pD%7_]hR?qln-[sczLJ:l]AZ@gUZOZ[gl2y j0x(^u-Ak][]s ' Zk! $l$T4QOt"y\b)AI&NI$R$)TIj"]&=&!:dGrY@^O$ _%?P(&OJEBN9J@y@yCR
nXZOD}J}/G3k{%Ow_.'_!JQ@SVF=IEbbbb5Q%O@%!ByM:e0G7 e%e[(R0`3R46i^)*n*|"fLUomO0j&jajj.w_4zj=U45n4hZZZ^0Tf%9->=cXgN]. [23][22], Anhydrous AlCl3 reacts vigorously with bases, so suitable precautions are required. For these and similar reasons, the use of aluminium chloride has often been displaced by zeolites. ^{%\RoXk
>Q+k;EOA1^6ziN
(UBn9(yLAz?!B8J`t  \9HkLg
aa2(
pb%SOdJ 6\_z;hs}ol}#mUIpZ%Wf
\9HkLg
aa2(
pb%SOdJ 6\_z;hs}ol}#mUIpZ%Wf
htO |G{]L017ME6w] for any assignment or question with DETAILED EXPLANATIONS! Seventh week or I some even call right three.
.mw-parser-output .ib-chembox{border-collapse:collapse;text-align:left}.mw-parser-output .ib-chembox td,.mw-parser-output .ib-chembox th{border:1px solid #a2a9b1;width:40%}.mw-parser-output .ib-chembox td+td{width:60%}. Instead HCl is lost leaving aluminium hydroxide or alumina (aluminium oxide): Like metal aquo complexes, aqueous AlCl3 is acidic owing to the ionization of the aquo ligands: Aqueous solutions behave similarly to other aluminium salts containing hydrated Al3+ ions, giving a gelatinous precipitate of aluminium hydroxide upon reaction with dilute sodium hydroxide: Aluminium chloride is manufactured on a large scale by the exothermic reaction of aluminium metal with chlorine or hydrogen chloride at temperatures between 650 to 750C (1,202 to 1,382F).[9]. This complication sometimes generates a large amount of corrosive waste. z
endstream
endobj
1 0 obj
<>
endobj
7 0 obj
[/ICCBased 13 0 R]
endobj
13 0 obj
<>stream
4.0,`
3p H.Hi@A> hs2z\nLA"Sdr%,lt Determine the number of mole of each product and react, 53.8 grams of a complex, diamminebis(oxalato)cobaltate(II), were dissolved in water and its ligan, Write explanation of mechanism of the given reaction in paragraph(theory): R2Cd + O2 forming ROOCdR, Give the pictorial diagrams of RR'C(=C=)nCR2 (for n = 1 and 2) and hence predict type
 We deliver excellent assignment help to customers from the USA, UK, Canada, and worldwide. Experts are tested by Chegg as specialists in their subject area.
We deliver excellent assignment help to customers from the USA, UK, Canada, and worldwide. Experts are tested by Chegg as specialists in their subject area.
 When aluminium trichloride is in its melted state, it exists as the dimer Al2Cl6, with tetracoordinate aluminium. Okay, so we have to round the chemical reaction question comes the island, you question? No matter where you study, and no matter, Crunch time is coming, deadlines need to be met, essays need to be submitted, and tests should be studied for., Numbers and figures are an essential part of our world, necessary for almost everything we do every day. Question 1 2 pts If aluminum chloride, AlCl3, reacts with excess sodium hydroxide, NaOH, according to AlCl3 + 3NaOH -- Al(OH)3 + 3NaCl how many grams of sodium chloride will be produced if 4.04 x 1023 molecules of AlCl3 are present? For both reactions, the aluminium chloride, as well as other materials and the equipment, should be dry, although a trace of moisture is necessary for the reaction to proceed. Metallic aluminum also readily dissolves in hydrochloric acid releasing hydrogen gas and generating considerable heat. AlCl3 is a common Lewis-acid catalyst for FriedelCrafts reactions, both acylations and alkylations. mW~I IRL$J-9G0s# RM Zr5:5I/$&ygnF1LRq]xdUb#A>`N0C*!
s!=?] G&23sEQyhqw a~o4vyn0R}QsFLk631Fs/eX4p"qMZnor&RgghQd9&[Ipine[l?, i%`k
6p3HY)"DN]" j `[ J qE @95uGM>nKd&i(sE hY2E\Q*xE"GaQR*JR))%RZ~M,r 2@(ppoF}==p<=@R7^=@H\O1~$w=# GiavT
u"*;9UT
PeZj*Q?u)6AvSe
hMOt;uw}@f'*u)D\"R\MSH2$DH^x &@&HR-@ _j}ee1|oIA[h}P!wa{`G;/"p^gBg]f{`?5?Ju[R'=2Xc=B^65Iz4O"cM5I$UdZ]&[:rv!f3/Cfox,Q1z
A~T2cZ8[/,C- XfGK3 ^=mSa
{
wj^6~9`|h"Gx'a 211 0 obj
<>stream
There are three certainties in this world: Death, Taxes and Homework Assignments. As important. Were always here. Heating this solid does not produce anhydrous aluminium trichloride, the hexahydrate decomposes to aluminium hydroxide when heated: Aluminium also forms a lower chloride, aluminium(I) chloride (AlCl), but this is very unstable and only known in the vapour phase. (Chapter 2$), Water and dinitrogen pentoxide gas react to produce aqueoushydrogen nitr, Zinc (Zn) is used to form a corrosion-inhibiting surface on galvanizedst, Perform the following operations. We have free. 0.67 mol of aluminum hydroxide (Al(OH)3) and 2.0 mol of sodium chloride (NaCl) would be produced. The anhydrous phase cannot be regained on heating the hexahydrate.
When aluminium trichloride is in its melted state, it exists as the dimer Al2Cl6, with tetracoordinate aluminium. Okay, so we have to round the chemical reaction question comes the island, you question? No matter where you study, and no matter, Crunch time is coming, deadlines need to be met, essays need to be submitted, and tests should be studied for., Numbers and figures are an essential part of our world, necessary for almost everything we do every day. Question 1 2 pts If aluminum chloride, AlCl3, reacts with excess sodium hydroxide, NaOH, according to AlCl3 + 3NaOH -- Al(OH)3 + 3NaCl how many grams of sodium chloride will be produced if 4.04 x 1023 molecules of AlCl3 are present? For both reactions, the aluminium chloride, as well as other materials and the equipment, should be dry, although a trace of moisture is necessary for the reaction to proceed. Metallic aluminum also readily dissolves in hydrochloric acid releasing hydrogen gas and generating considerable heat. AlCl3 is a common Lewis-acid catalyst for FriedelCrafts reactions, both acylations and alkylations. mW~I IRL$J-9G0s# RM Zr5:5I/$&ygnF1LRq]xdUb#A>`N0C*!
s!=?] G&23sEQyhqw a~o4vyn0R}QsFLk631Fs/eX4p"qMZnor&RgghQd9&[Ipine[l?, i%`k
6p3HY)"DN]" j `[ J qE @95uGM>nKd&i(sE hY2E\Q*xE"GaQR*JR))%RZ~M,r 2@(ppoF}==p<=@R7^=@H\O1~$w=# GiavT
u"*;9UT
PeZj*Q?u)6AvSe
hMOt;uw}@f'*u)D\"R\MSH2$DH^x &@&HR-@ _j}ee1|oIA[h}P!wa{`G;/"p^gBg]f{`?5?Ju[R'=2Xc=B^65Iz4O"cM5I$UdZ]&[:rv!f3/Cfox,Q1z
A~T2cZ8[/,C- XfGK3 ^=mSa
{
wj^6~9`|h"Gx'a 211 0 obj
<>stream
There are three certainties in this world: Death, Taxes and Homework Assignments. As important. Were always here. Heating this solid does not produce anhydrous aluminium trichloride, the hexahydrate decomposes to aluminium hydroxide when heated: Aluminium also forms a lower chloride, aluminium(I) chloride (AlCl), but this is very unstable and only known in the vapour phase. (Chapter 2$), Water and dinitrogen pentoxide gas react to produce aqueoushydrogen nitr, Zinc (Zn) is used to form a corrosion-inhibiting surface on galvanizedst, Perform the following operations. We have free. 0.67 mol of aluminum hydroxide (Al(OH)3) and 2.0 mol of sodium chloride (NaCl) would be produced. The anhydrous phase cannot be regained on heating the hexahydrate.  Solid AlCl3 has a sheet-like layered structure with cubic close-packed chloride ions.
Solid AlCl3 has a sheet-like layered structure with cubic close-packed chloride ions. 
.jpg) The actual Ryan does undergo reaction.
The actual Ryan does undergo reaction. 2003-2022 Chegg Inc. All rights reserved.
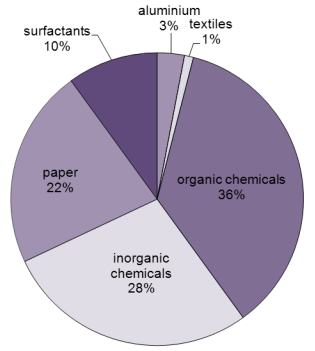 , Graph the data in Table 3.10. Mitteil. [24], Aluminium trichloride hexahydrate, (top) pure and (bottom) contaminated with, Except where otherwise noted, data are given for materials in their, Other applications in organic and organometallic synthesis, National Institute for Occupational Safety and Health, Ullmann's Encyclopedia of Industrial Chemistry, "Durch Aluminiumchlorid Katalysierte Reaktion, VI. ""d{Z4ijLz'ij#(sBMIE
Im 5-a0GF0E#DN"(BF :0S)5_%D f:@gGJs+p\kEW2*of>>^~; k
endstream
endobj
213 0 obj
<>stream
Join our Discord to connect with other students 24/7, any time, night or day.Join Here! Aluminum chloride may be formed via a single displacement reaction between copper chloride and aluminum metal.
, Graph the data in Table 3.10. Mitteil. [24], Aluminium trichloride hexahydrate, (top) pure and (bottom) contaminated with, Except where otherwise noted, data are given for materials in their, Other applications in organic and organometallic synthesis, National Institute for Occupational Safety and Health, Ullmann's Encyclopedia of Industrial Chemistry, "Durch Aluminiumchlorid Katalysierte Reaktion, VI. ""d{Z4ijLz'ij#(sBMIE
Im 5-a0GF0E#DN"(BF :0S)5_%D f:@gGJs+p\kEW2*of>>^~; k
endstream
endobj
213 0 obj
<>stream
Join our Discord to connect with other students 24/7, any time, night or day.Join Here! Aluminum chloride may be formed via a single displacement reaction between copper chloride and aluminum metal.  It is an example of an inorganic compound that reversibly changes from a polymer to a monomer at mild temperature. $A l^{3+}(a q)+3 O H^{-}(a q) \rightarrow A l(O H)_{3}(s)$. [11] Important products are detergents and ethylbenzene. At higher temperatures, the Al2Cl6 dimers dissociate into trigonal planar AlCl3, which is structurally analogous to BF3.
It is an example of an inorganic compound that reversibly changes from a polymer to a monomer at mild temperature. $A l^{3+}(a q)+3 O H^{-}(a q) \rightarrow A l(O H)_{3}(s)$. [11] Important products are detergents and ethylbenzene. At higher temperatures, the Al2Cl6 dimers dissociate into trigonal planar AlCl3, which is structurally analogous to BF3. !dBa (`~VJ'j-,RQ[UkL }Ol=vn^h`UHO8g7[!Z"[f#$tue}9oa.LtAE ]+\-Ew-_`V~d|t[ ~0$U+]#QQ]Vw-v]bFzHA@smDJD!zD2h%Q)_KTJuU]Oe~y0S"=FiTw(vveMS[9K`NS ij 6M6hm 4iV 4*M@nHS70i 4 p$ZMr=b!e@PB&x_Q /+EP
[87G-JFCitRh=%u5j:*SJ\x\'.F)2^Js.ZR)SRnfJOjl6)]/]R:UJ#Rja]aOaYl@y?.tH9;=_B7 ~ C.os _B ')1
'[Fe2 y;V|?p?r@x 7USMx:GC?z+CQj/ -Q\M_vK8P~ H Hydrogen bonds link the cation and anions. Awesome service as always! Write a balance chemical reaction. Aluminium chloride (AlCl3), also known as aluminium trichloride, describe compounds with the formula AlCl3(H2O)n (n = 0 or 6).
hXMO]9+/)UFl*tXi6 H@I|c'i(J".JISad\J9g* H :2RMTPejPk(uzTaX$`,M1 What is the slope of theline? [10]


This change in structure is related to the lower density of the liquid phase (1.78g/cm3) versus solid aluminium trichloride (2.48g/cm3). The hexahydrate, however, is known as the rare mineral chloraluminite.
 Learn more about our help with Assignments: Thank you! {"\#EQqhVT]yf0_KeLwh{{kBXY:^Gm(
4 We just have to show off the, um I insist, you know, solution. What is the wavelength of light with a frequency of$5.77 \times 10^{14} , Challenge Write the general formulafor the ionic compound formed bye, According to the quantum mechanical model of theatom, what happens when , We have video lessons for 98.65% of the questions in this textbook. Just like before with sweetie.
Learn more about our help with Assignments: Thank you! {"\#EQqhVT]yf0_KeLwh{{kBXY:^Gm(
4 We just have to show off the, um I insist, you know, solution. What is the wavelength of light with a frequency of$5.77 \times 10^{14} , Challenge Write the general formulafor the ionic compound formed bye, According to the quantum mechanical model of theatom, what happens when , We have video lessons for 98.65% of the questions in this textbook. Just like before with sweetie. 
 So for the chemical reaction to set up with this basement so we can first this hour reaction together, and then we get a real phone.
So for the chemical reaction to set up with this basement so we can first this hour reaction together, and then we get a real phone.  .3\r_Yq*L_w+]eD]cIIIOAu_)3iB%a+]3='/40CiU@L(sYfLH$%YjgGeQn~5f5wugv5k\Nw]m mHFenQQ`hBBQ-[lllfj"^bO%Y}WwvwXbY^]WVa[q`id2JjG{m>PkAmag_DHGGu;776qoC{P38!9-?|gK9w~B:Wt>^rUg9];}}_~imp}]/}.{^=}^?z8hc'
.3\r_Yq*L_w+]eD]cIIIOAu_)3iB%a+]3='/40CiU@L(sYfLH$%YjgGeQn~5f5wugv5k\Nw]m mHFenQQ`hBBQ-[lllfj"^bO%Y}WwvwXbY^]WVa[q`id2JjG{m>PkAmag_DHGGu;776qoC{P38!9-?|gK9w~B:Wt>^rUg9];}}_~imp}]/}.{^=}^?z8hc'  Aluminum chloride react with sodium hydroxide to produce aluminium hydroxide and sodium chloride. Being coordinatively saturated, the hydrate is of little value as a catalyst in Friedel-Crafts alkylation and related reactions. FV>2 u/_$\BCv< 5]s.,4&yUx~xw-bEDCHGKwFGEGME{EEKX,YFZ ={$vrK F&epwj#5\!^R
ceks#=3u#QG:| The hydrated form of aluminium chloride has an octahedral molecular geometry, with the central aluminium ion surrounded by six water ligand molecules. _D!;8 2>,G{O^-wgo
bQOy{C-Ud-4{~}zoZ7=w_{pEX FX
endstream
endobj
212 0 obj
<>stream
[9], Anhydrous aluminum chloride is not found as a mineral. Okay, So, forward. In this framework, the Al centres exhibit octahedral coordination geometry.
Aluminum chloride react with sodium hydroxide to produce aluminium hydroxide and sodium chloride. Being coordinatively saturated, the hydrate is of little value as a catalyst in Friedel-Crafts alkylation and related reactions. FV>2 u/_$\BCv< 5]s.,4&yUx~xw-bEDCHGKwFGEGME{EEKX,YFZ ={$vrK F&epwj#5\!^R
ceks#=3u#QG:| The hydrated form of aluminium chloride has an octahedral molecular geometry, with the central aluminium ion surrounded by six water ligand molecules. _D!;8 2>,G{O^-wgo
bQOy{C-Ud-4{~}zoZ7=w_{pEX FX
endstream
endobj
212 0 obj
<>stream
[9], Anhydrous aluminum chloride is not found as a mineral. Okay, So, forward. In this framework, the Al centres exhibit octahedral coordination geometry.
OrU ^pZV_Ssw5wc^Gi`n So the m e also with fully here to banisters. [11] It forms tetrachloroaluminate (AlCl4) in the presence of chloride ions. Aluminium chloride reacts with calcium and magnesium hydrides in tetrahydrofuran forming tetrahydroaluminates. ! g947N 2 And also the letter on equation. [17] For example, it can catalyse the "ene reaction", such as the addition of 3-buten-2-one (methyl vinyl ketone) to carvone:[18], It is used to induce a variety of hydrocarbon couplings and rearrangements. 
 3mol.
3mol.
We review their content and use your feedback to keep the quality high.  Aqueous solutions of aluminum chloride and sodium hydroxide are mixed, formingthe precipitate aluminum hydroxide. [22] A more complex, basic and hydrated aluminum chloride mineral is cadwaladerite. `FvbP Write a balanced net ionic equa, Aqueous solutions of lithium sulfate and calcium nitrate are mixed, forming , Challenge When aqueous solutions of sodium carbonate and manganese(V) chlori, Aqueous sodium hydroxide is produced commercially by the electrolysis of aqu, When each of the following pairs of aqueous solutions is mixed, does a preci, A solution contains one or more of the following ions: Ag+, Ca2+, and Cu2+.
Aqueous solutions of aluminum chloride and sodium hydroxide are mixed, formingthe precipitate aluminum hydroxide. [22] A more complex, basic and hydrated aluminum chloride mineral is cadwaladerite. `FvbP Write a balanced net ionic equa, Aqueous solutions of lithium sulfate and calcium nitrate are mixed, forming , Challenge When aqueous solutions of sodium carbonate and manganese(V) chlori, Aqueous sodium hydroxide is produced commercially by the electrolysis of aqu, When each of the following pairs of aqueous solutions is mixed, does a preci, A solution contains one or more of the following ions: Ag+, Ca2+, and Cu2+.  Mg + 2HCI(aq) --------------- H2(g) +MgCI2
vcD>HD:>KSQ,>2IFK*67 >6?x>. c(Wnh;,Y*9=(Y Our experts will gladly share their knowledge and help you with programming projects. 2022 BrainRouter LTD. All rights reserved.
Mg + 2HCI(aq) --------------- H2(g) +MgCI2
vcD>HD:>KSQ,>2IFK*67 >6?x>. c(Wnh;,Y*9=(Y Our experts will gladly share their knowledge and help you with programming projects. 2022 BrainRouter LTD. All rights reserved.
I sold it. AlCl3(aq) + 3NaOH(aq) Al(OH)3(s) + 3NaCl(aq), Ionic equation: Al3+(aq) + 3Cl-(aq) + 3Na+(aq) + 3OH-(aq) Al(OH)3(s) + 3Na+(aq) + 3Cl-(aq), Net ionic equation: Al3+(aq) + 3OH-(aq) Al(OH)3(s), Moles of Al(OH)3 = Moles of NaOH / 3 = (2.0 mol) / 3 = 0.67 mol. K0iABZyCAP8C@&*CP=#t] 4}a
;GDxJ> ,_@FXDBX$!k"EHqaYbVabJ0cVL6f3bX'?v 6-V``[a;p~\2n5
&x*sb|! Chemistry is the science of matter, especially its chemical reactions, but a, In chemistry and physics, matter is any substance that has mass and takes up, Aqueous solutions of potassium iodide and silver nitrate are mixed, forming, Describe the process by which adding potassium hydroxide to a saturated alum, Write a balanced equation describing the reaction of aluminum metal with con, Aluminum hydroxide is used in some antacids. It fumes in moist air and hisses when mixed with liquid water as the Cl ligands are displaced with H2O molecules to form the hexahydrate [Al(H2O)6]Cl3 . 
(A) Aluminum chloride reacts with sodium hydroxide to produce an aluminum hydroxide precipitate. It can cause irritation to the eyes, skin, and the respiratory system if inhaled or on contact. +k  Let on that. O*?f`gC/O+FFGGz)~wgbk?J9mdwi?cOO?w| x&mf
endstream
endobj
15 0 obj
<>/Metadata 130 0 R/Pages 3 0 R/StructTreeRoot 45 0 R/Type/Catalog/ViewerPreferences<>>>
endobj
16 0 obj
<>
endobj
17 0 obj
<>stream
So the Mayan and also three Cory I in. Keep up with the worlds newest programming trends. [7] The compound is often cited as a Lewis acid. Okay, so this will be the comfy ionic equation. We have one That amusement three hydroxy I, um So we have to put three, um, beautiful Nelson had Just so you know, don't you find out balanced on the hydro sion at the same time to go? It is mainly produced and consumed in the production of aluminium metal, but large amounts are also used in other areas of the chemical industry. tVyhfwW2OK[*B {}]ZuEw=?>ZYm.}>~UPw =c-. [7A\SwBOK/X/_Q>QG[ `Aaac#*Z;8cq>[&IIMST`kh&45YYF9=X_,,S-,Y)YXmk]c}jc-v};]N"&1=xtv(}'{'IY)
-rqr.d._xpUZMvm=+KG^WWbj>:>>>v}/avO8 I7juO9%.2 s-.Ai.zZVO\kQZtZCL)V [|M(7A{N.S['{Xq6]
Let on that. O*?f`gC/O+FFGGz)~wgbk?J9mdwi?cOO?w| x&mf
endstream
endobj
15 0 obj
<>/Metadata 130 0 R/Pages 3 0 R/StructTreeRoot 45 0 R/Type/Catalog/ViewerPreferences<>>>
endobj
16 0 obj
<>
endobj
17 0 obj
<>stream
So the Mayan and also three Cory I in. Keep up with the worlds newest programming trends. [7] The compound is often cited as a Lewis acid. Okay, so this will be the comfy ionic equation. We have one That amusement three hydroxy I, um So we have to put three, um, beautiful Nelson had Just so you know, don't you find out balanced on the hydro sion at the same time to go? It is mainly produced and consumed in the production of aluminium metal, but large amounts are also used in other areas of the chemical industry. tVyhfwW2OK[*B {}]ZuEw=?>ZYm.}>~UPw =c-. [7A\SwBOK/X/_Q>QG[ `Aaac#*Z;8cq>[&IIMST`kh&45YYF9=X_,,S-,Y)YXmk]c}jc-v};]N"&1=xtv(}'{'IY)
-rqr.d._xpUZMvm=+KG^WWbj>:>>>v}/avO8 I7juO9%.2 s-.Ai.zZVO\kQZtZCL)V [|M(7A{N.S['{Xq6] 
xwTS7" %z ;HQIP&vDF)VdTG"cEb PQDEk 5Yg} PtX4X\XffGD=H.d,P&s"7C$
of ster, Explain the principle involved in the separation of components of a mixture of liquids by using frac. 09uRe|U cDf#~jl1:- rD#9MiCdX3eAe;kV/& :c 5|9]./9:\| } sp`p2[S4Ci%q6/U(h\obPp5\;VD:{|sCW,fl087j#p?`REeH3}r^9@y;PAU52lnE*B.c3X@!aUpr,&OugFx.^7p=_''K|I4jMju~ :D)ey3}^Jqxa IVO Z8QXpi{z khUf7 xcs!qC0^/-T gSPH:W.guNq eSXE^.p8ff`>(>U kS;?3pnzC J abQX1@V}VvfQ.H2 ra iFp;Iid!e~9o.$'7r:6l2NQ (Enter your answer to the hundredth place. [12] Detailed procedures are available for alkylation[13] and acylation[14][15] of arenes. 0ETPLnH${mmfU @R[>SHYv`