Since catalysts do not change in quantity or  When hydrogen peroxide acts as an oxidizing agent, it gives up one of the oxygen atoms, leaving behind water as a byproduct. The bubbles happen when the hydrogen peroxide reacts with your living cells causing them to break down into water and oxygen. Oxygen can be prepared from the decomposition of hydrogen peroxide: Manganese dioxide is the catalyst in this reaction. The general equation for making hydrogen peroxide is to simply combine hydrogen with oxygen and hydrogen peroxide is formed. The melting point for hydrogen peroxide is -0.4 degrees Celsius, and the boiling point is 150 degrees Celsius. What is the other product of this reaction? column. It isn't shown on the image but this hydrogen is on a metal catalyst. Constant temperature T The, The IUPAC name of an element with atomic number 119 is. The structure of hydrogen peroxide is non-planar. Amongst the following which one will have maximum 'lone pair - lone pair' electron repulsions.
When hydrogen peroxide acts as an oxidizing agent, it gives up one of the oxygen atoms, leaving behind water as a byproduct. The bubbles happen when the hydrogen peroxide reacts with your living cells causing them to break down into water and oxygen. Oxygen can be prepared from the decomposition of hydrogen peroxide: Manganese dioxide is the catalyst in this reaction. The general equation for making hydrogen peroxide is to simply combine hydrogen with oxygen and hydrogen peroxide is formed. The melting point for hydrogen peroxide is -0.4 degrees Celsius, and the boiling point is 150 degrees Celsius. What is the other product of this reaction? column. It isn't shown on the image but this hydrogen is on a metal catalyst. Constant temperature T The, The IUPAC name of an element with atomic number 119 is. The structure of hydrogen peroxide is non-planar. Amongst the following which one will have maximum 'lone pair - lone pair' electron repulsions.
The mass of oxygen compared to air can be investigated in a laboratory. Hydrogen peroxide acts as a reducing agent in this reaction. So, the correct answer is option C; manganese dioxide does not change in quantity or structure when used in the preparation The order of energy absorbed which is responsible for the color of complexes, The product formed from the following reaction sequence is. Hydrogen peroxide in both acidic and basic medium acts as an oxidizing as well as a reducing agent. Its like a teacher waved a magic wand and did the work for me. Hydrogen peroxide is a strong oxidizing agent. H2O2 is manufactured by followed by vacuum distillation. When hydrogen peroxide decomposes in the presence of manganese dioxide, oxygen is formed: The oxygen would escape in bubbles, making the hydrogen peroxide bubble and fizz. Reaction: H2O2 H2O + [O] Nascent oxygen. It is also used as a propellant in rockets.o Then, we see that the resulting anthraquinone can be exposed to hydrogen to reform the hydroquinone, and the reaction cycles back to the beginning. An error occurred trying to load this video. above on a water bath preferably under reduced pressure using fractionating We also can test if oxygen gas is soluble (dissolves) in water by placing a cylinder of gaseous oxygen upside down in water. Given below are two statements: one is labelled as Assertion (A) and the other is labelled as Reason (R). flashcard sets, {{courseNav.course.topics.length}} chapters | copyright 2003-2022 Study.com. It is used in the laboratory for detecting the presence of chromium, titanium, etc. (d) The method of collecting oxygen is downward displacement of water. This structure is not in a planar molecule but instead has a twisted symmetry. Molarity of hydrogen peroxide = 30.36/34 = 0.89 M. Also Read: What is Hydrogen Peroxide Used For. the image below. Laboratory preparation Earths atmosphere is The impurities like organic material or metallic We can then observe the level of the water in the cylinder: When we do this experiment, the water level rises only a small amount. 4. This tells us that gaseous oxygen scarcely dissolves When hydrogen peroxide is broken down in the presence of a black powder called manganese dioxide, new substances (products) 2. In this explainer, we have learned how to prepare oxygen gas and test some of its properties. This is an example of a decomposition reaction. sodium peroxide. Hydrogen peroxide can be an oxidizing agent or a reducing agent. Traces of H2O2 are found in atmosphere and How to Determine the Concentration of Hydrogen Peroxide?
hydrogenperoxideoxygengas+inthepresenceofmanganesedioxide. It should also be kept away from dust particles because dust can induce explosive decomposition of this compound. However, the boiling point is much higher than water, at 150 degrees Celsius. The strength of a sample of hydrogen peroxide | Urea Molecular Structure & Formula. volume of H2O2 gives on heating. 2HSO4(aq) [Electrolysis] HO3SOOSO3H(aq) [Hydrolysis] 2HSO4(aq)+2H+(aq)+H2O2(aq), 2HSO4 H2S2O8+ 2e Peroxodi Sulphuric Acid [Marshalls acid], H2S2O8+ H2O H2SO5+ H2SO4 Peroxomono Sulphuric Acid [Caros acid], Auto-Oxidation Method of Preparation of H2O2. feathers. Developed by Therithal info, Chennai. Fady and Lobna are talking about the oxygen produced when hydrogen peroxide decomposes. SAT Subject Test Physics: Tutoring Solution, Physical Science Curriculum Resource & Lesson Plans, ILTS Science - Environmental Science (112): Test Practice and Study Guide, SAT Subject Test Chemistry: Practice and Study Guide, ILTS Science - Chemistry (106): Test Practice and Study Guide, NY Regents Exam - Chemistry: Test Prep & Practice, High School Chemistry: Homework Help Resource, High School Physical Science: Tutoring Solution, NY Regents Exam - Chemistry: Tutoring Solution, NY Regents Exam - Physics: Help and Review, NY Regents Exam - Physics: Tutoring Solution, High School Physics: Homework Help Resource, Create an account to start this course today. Hydrogen peroxide has each oxygen connected to the other oxygen and to one of the hydrogen atoms. Pure H2O2 is unstable and decomposes on standing. and is used in bleaching delicate things like hair, wool, silk ivory and Oxidation is when more carbon-oxygen bonds are formed. materials like silk, wool, hair which will be destroyed by chlorine, are Find the emf of the cell in which the following reaction takes place at, The given graph is a representation of kinetics of a reaction. In this image, you can see that first we have hydroquinone.
Delicate If oxygen dissolves in the water, the water level rises, as the volume of oxygen gas decreases. Therefore, Fady is incorrect and only Lobna is correct. Oxygen can be prepared in a laboratory from a liquid called hydrogen peroxide. Put your understanding of this concept to test by answering a few MCQs. | Differentiated Instruction Resources, Customer Service Manager Skills & Training, High School US History Syllabus Resource & Lesson Plans, High School Trigonometry: Homework Help Resource, NMTA Elementary Education Subtest II (103): Practice & Study Guide, The Medieval Warm Period: Help and Review, Quiz & Worksheet - Lexically Ambiguous Sentences, Quiz & Worksheet - Ribosomes & Protein Synthesis, Quiz & Worksheet - David Wechsler on Intelligence, Quiz & Worksheet - Luddites During the Industrial Revolution, Motivational Tools for Students: Techniques & Examples, What Is Stomatitis? b) Write a balanced equation in which hydrogen peroxide reduces perman, Dr. Goodchem's favorite student made a new compound H_2O_2 Dr. student noted that the compound hat some of the same atoms as H_2O and is convinced that it would have the same physical and chemical pro, Select all of the true statements from the list below. Insert a burning match into the lower beaker. 3. (At anode), Concentration of Hydrogen peroxide can be found in glow sticks and rocket fuel, and can be used as an explosive or as a bleach for hair, paper, or flour. Get unlimited access to over 84,000 lessons. Collecting oxygen in this way would not be possible if oxygen dissolved easily in water. The bond angle between each oxygen and hydrogen is 102 degrees. The following diagram will clearly show what an open book structure means. Oxygen helps with burning. of dilute acid on barium peroxide. feathers. The following reaction will clarify this: BaO2.8H2O(s) + H2SO4(aq) BaSO4(s) + H2O2(aq) + 8H2O(l). Inorganic Chemistry Overview & Examples | What is Inorganic Chemistry? This would not be possible if oxygen dissolved in water. Ozone on reaction with hydrogen peroxide gives oxygen and water. It melts at 272.4 K and has a boiling point of 423 K (extrapolated). bleaching agent, powerful but harmless disinfectant and germicide. Q5) Compare the three main components of air i.e. are formed. So the melting point is very similar to that of water (which is 0 degrees Celsius), but the boiling point is much higher than the boiling point of water (which is 100 degrees Celsius). Therefore, H2O2 is stored in wax-lined glass or plastic containers and kept in dark. What experimental error usually occur in the reaction of hydrogen peroxide and bleach experiment? It acts as a bleaching agent and is also used as a disinfectant. in certain plants. To unlock this lesson you must be a Study.com Member. Try refreshing the page, or contact customer support. Kl is not a catalyst for the decomposition of hydrogen peroxide. On heating when water and oxygen are formed. as an electron acceptor. Laura has a Masters of Science in Food Science and Human Nutrition and has taught college Science. Class 7 It is in the air we breathe, the water we drink, and the food we eat. a mixture of gases that surround Earth. Hydrogen peroxide is prepared by the electrolysis of 30% ice-cold H2SO4. H2O2 + 2H+ + 2e-
water in the cylinder. Electronic Displacements in Covalent Bonds, Dobereiner's Law of Triads & Newlands' Law of Octaves, Oxidation of Alcohols | Reagents, Mechanism & Reaction, Common Ion Effect | Common Ion Chart, Effect on Solubility & Examples, De Broglie Equation Overview, Hypothesis & Examples | Wave Equation Formula, What is a Chemical Property? hydrogenperoxide solution. bleached with H. It destroys bacteria and hence it is used as an
of hydrogen peroxide.
Q5) Compare the three main components of air i.e. are formed. So the melting point is very similar to that of water (which is 0 degrees Celsius), but the boiling point is much higher than the boiling point of water (which is 100 degrees Celsius). Therefore, H2O2 is stored in wax-lined glass or plastic containers and kept in dark. What experimental error usually occur in the reaction of hydrogen peroxide and bleach experiment? It acts as a bleaching agent and is also used as a disinfectant. in certain plants. To unlock this lesson you must be a Study.com Member. Try refreshing the page, or contact customer support. Kl is not a catalyst for the decomposition of hydrogen peroxide. On heating when water and oxygen are formed. as an electron acceptor. Laura has a Masters of Science in Food Science and Human Nutrition and has taught college Science. Class 7 It is in the air we breathe, the water we drink, and the food we eat. a mixture of gases that surround Earth. Hydrogen peroxide is prepared by the electrolysis of 30% ice-cold H2SO4. H2O2 + 2H+ + 2e-
water in the cylinder. Electronic Displacements in Covalent Bonds, Dobereiner's Law of Triads & Newlands' Law of Octaves, Oxidation of Alcohols | Reagents, Mechanism & Reaction, Common Ion Effect | Common Ion Chart, Effect on Solubility & Examples, De Broglie Equation Overview, Hypothesis & Examples | Wave Equation Formula, What is a Chemical Property? hydrogenperoxide solution. bleached with H. It destroys bacteria and hence it is used as an
of hydrogen peroxide.  Gas 3 has odor, so it cannot be oxygen gas. is obtained by reacting BaO2 with an acid BaO2 + H2SO4 -- > Ba SO4 + H2O2. Manganese dioxide is a catalyst used in the preparation of oxygen from hydrogen peroxide. So, the answer is option D; after one minute, all of the oxygen will be in beaker 2 because it is heavier than air. It is also used as a propellant in rockets.o
- Formula, Production & Uses, AP EAMCET AM (Agriculture & Medical) Flashcards, High School Physics Curriculum Resource & Lesson Plans, Physics 101 Syllabus Resource & Lesson Plans, Middle School Physical Science Curriculum Resource & Lesson Plans, 7th Grade Physical Science: Enrichment Program, 6th Grade Physical Science: Enrichment Program, Physical Geology Syllabus Resource & Lesson Plans, NY Regents Exam - Earth Science: Tutoring Solution, ILTS Health Education (211): Test Practice and Study Guide, What Are Peroxide Antiseptics?
Gas 3 has odor, so it cannot be oxygen gas. is obtained by reacting BaO2 with an acid BaO2 + H2SO4 -- > Ba SO4 + H2O2. Manganese dioxide is a catalyst used in the preparation of oxygen from hydrogen peroxide. So, the answer is option D; after one minute, all of the oxygen will be in beaker 2 because it is heavier than air. It is also used as a propellant in rockets.o
- Formula, Production & Uses, AP EAMCET AM (Agriculture & Medical) Flashcards, High School Physics Curriculum Resource & Lesson Plans, Physics 101 Syllabus Resource & Lesson Plans, Middle School Physical Science Curriculum Resource & Lesson Plans, 7th Grade Physical Science: Enrichment Program, 6th Grade Physical Science: Enrichment Program, Physical Geology Syllabus Resource & Lesson Plans, NY Regents Exam - Earth Science: Tutoring Solution, ILTS Health Education (211): Test Practice and Study Guide, What Are Peroxide Antiseptics?
H2O2 bleaches silk, wool, cotton, hair. It is a colourless liquid and is used in aqueous solution for safety reasons. In the pure state, hydrogen peroxide is almost colourless (very pale blue) liquid.
485 lessons Insert a burning match into the upper beaker. But it's still a disinfectant for many different surfaces and objects, including surgical instruments. because it is than air. | {{course.flashcardSetCount}} Moist silver oxide, acidified KMnO4, So, the correct answer is water; it is the other product of the reaction. By careful evaporation of the solution obtained What happens to manganese dioxide in this reaction? Oxygen does not dissolve in water, but it does above on a water bath preferably under reduced pressure using fractionating The Kjeldahl's method for the estimation of nitrogen can be used to estimate the amount of nitrogen in which one of the following compounds? We also know that a catalyst is a substance whose quantity and structure do not change during a chemical reaction. Click Start Quiz to begin! This fascinating liquid is called hydrogen peroxide. Peroxodisulphate is then hydrolyzed to get hydrogen peroxide. Please contact your portal admin. It may look empty, but in fact, it contains oxygen gas. {{courseNav.course.mDynamicIntFields.lessonCount}} lessons lessons in math, English, science, history, and more. By distillation under reduced pressure at Oxygen is scarcely soluble in water. Hydrogen peroxide is used for stain removal, as a cleanser and as a disinfectant. solution is expressed in terms of the volumes of oxygen at S.T.P that one Q2) Name the important scientists and their studies which led to the discovery of the components of Q3) Explain Lavoisers experiment which provided evidence to the discovery of components in air. in water. To calculate the molarity, Let us consider 10 Vol H2O2. When two gases are mixed, the heavier gas will fall to the bottom. volume of H, Due to its oxidizing property, it is a valuable It turns filter paper containing black stains of. 10 Vol H2O2 means 1 mL of H2O2 gives 10 mL of O2 at STP. It is used as an antiseptic for washing wounds. I would definitely recommend Study.com to my colleagues. We've never actually been able to boil hydrogen peroxide because, as we heat up hydrogen peroxide, it explodes and decomposes into water and oxygen. A catalyst is a substance whose quantity and structure do not change during a chemical reaction. The hydrogens, which were on the oxygen, now combine with the oxygen to form hydrogen peroxide. Having collected oxygen gas, we can investigate its properties. If the oxygen does not dissolve in the water, the water level will stay the same. Create your account, 57 chapters | (b) Catalyst used is manganese dioxide, it increases the speed of decomposition of H2O2. Which amongst the following is incorrect statement? the molar mass of H2O2 is 34.0147g/mol and the density of the solution is 1.000 g/ml. Hydrogen peroxide is the simplest kind of peroxide available (oxygen-oxygen single bond). By the action of dilute sulphuric acid on 30% solution of H2O2 is obtained by this process. -- > 2 H2O, It oxidizes ferrous salts int2Fe2+ + 2H+ + H2O2 --> 2 Fe3+ + 2H2O. The following reactions will give a clear picture: It bleaches by oxidation. H2O2 is a colourless, odourless, syrupy liquid Hydrogen Peroxide: Preparation, Properties & Structure, Heisenberg Uncertainty Principle: Definition & Equation, Valence Bond Theory of Coordination Compounds, Heterogeneous vs. Homogenous Catalysts | Overview, Differences & Examples, What is Urea? Plus, get practice tests, quizzes, and personalized coaching to help you Generally, it's prepared through oxidation of a hydroquinone. Additional Adjustments to Income for Taxes, Microscopic Procedures for Direct Examination of Parasite Specimens, Quiz & Worksheet - Features & Genres of Dance, Quiz & Worksheet - Death on the Nile Literary Elements, Flashcards - Real Estate Marketing Basics, Flashcards - Promotional Marketing in Real Estate, Science Worksheets | Printable Science Worksheets for Teachers, What is Differentiated Instruction? One of the products made when hydrogen peroxide breaks down is oxygen. It is used in the production of epoxides, inorganic chemicals like sodium perborate. Hydrogen peroxide is a bubbly, antimicrobial agent used as an environmentally friendly disinfectant. | Meaning, Types, & List of Examples, The Solvay Process: Process, Products & Environmental Issues, Nitric Acid Formula and Structure | Chemical Formula of Nitric Acid. Therefore, when oxygen and air are mixed, oxygen will fall to the bottom. (c) State the word equation for the reaction involving the above preparation of oxygen. 1.
Here. The physical properties of hydrogen peroxide are: The oxidation state of oxygen in hydrogen peroxide is -1. Catalysts speed up a It would sting but at the same time it was very intriguing because it would bubble and fizz. It's also used as a common alternative medicine treatment. Q4) Tabulate the various components of air, including the components with variable composition. The oxygen displaces the A hydroquinone is an aromatic compound derived from benzene, which acts as the source of hydrogens in the hydrogen peroxide. ferric salts . displace water. Heating samples to 100 degrees C f. Calculate the mass percent of the hydrogen peroxide in the old bottle. Let us have a look at the various methods of preparation for hydrogen peroxide. Traces of H2O2 are found in atmosphere and in certain plants. The experiment can be carried out in the laboratory using the equipment and steps shown below. formed. Look at this jar. - Definition, Types & Uses, Prokaryotic Algae Cells: Function, Definition & Features, Ascaris Parasitic Worms: Phylum & Classification, Ascaris Worms: Anatomy & Digestive Systems, Phylum Annelida Circulatory & Nervous Systems, Phylum Annelida Digestive & Respiratory Systems, Porifera Respiration & Respiratory System, Tapeworms in Humans: Symptoms & Treatment, TExES Science of Teaching Reading (293): Practice & Study Guide, Curriculum & Assessment in Music Education, Planned Value vs. Earned Value in Project Management, Difference Between There, Their & They're. column. Hydrogen peroxide is prepared in the laboratory by: Which one is not correct mathematical equation for Dalton's Law of partial pressure ? Gas 2 has color, so it cannot be oxygen gas. Identify the incorrect statement from the following. of oxygen from hydrogen peroxide. Since it does break down into two very harmless compounds, water and oxygen, it's seen as an environmentally friendly antimicrobial. The incorrect statement regarding chirality is : Which statement regarding polymers is not correct? We can investigate how heavy oxygen is compared to air using these steps: When we do this, we see that the match glows more strongly in the lower beaker. Study Material, Lecturing Notes, Assignment, Reference, Wiki description explanation, brief detail, 11th 12th std standard Class Organic Inorganic Physical Chemistry Higher secondary school College Notes : Hydrogen peroxide: Preparation, Properties, reactions, Uses |, Hydrogen peroxide: Preparation, Properties, reactions, Uses. The presence of oxygen makes a match glow brighter. One of Gas 1 has color and odor, so it cannot be oxygen gas. It destroys bacteria and hence it is used as an materials like silk, wool, hair which will be destroyed by chlorine, are (b) Name the catalyst used in the preparation and state its function. Terms and Conditions, Gadolinium has a low value of third ionisation enthalpy because of, Which one of the following is not formed when acetone reacts with 2-pentanone in the presence of dilute. Three particles have speeds of 2u10u and 11u Which class 11 chemistry JEE_Main, At very high pressure the compressibility factor of class 11 chemistry JEE_Main, Which of the following is not a gaseous fuel A CNG class 11 chemistry JEE_Main, An octahedral complex with molecular composition M5NH3ClSO4 class 11 chemistry JEE_Main, Amongst LiCl RbCl BeCl2 and MgCl2the compounds with class 11 chemistry JEE_Main, In which of the following pairs 1st is more stable class 11 chemistry JEE_Main, Epipetalous and syngenesious stamens occur in aSolanaceae class 11 biology CBSE, Differentiate between homogeneous and heterogeneous class 12 chemistry CBSE, Cl P Cl bond angles in PCl5 molecule is A 120 circ class 11 chemistry CBSE, Two vectors of equal magnitude have a resultant equal class 11 physics CBSE, A magnetic moment of 173BM will be shown by which one class 11 chemistry CBSE, Which of the following cannot be prepared by Sandmeyers class 11 chemistry CBSE, The maximum number of possible oxidation states of class 12 chemistry CBSE, What would happen to the life of a cell if there was class 11 biology CBSE, Name the members of the lanthanoid series which exhibit class 12 chemistry CBSE, CBSE Previous Year Question Paper for Class 10, CBSE Previous Year Question Paper for Class 12. I feel like its a lifeline. ions, may catalyse its explosive decomposition. Role of hydrogen peroxide in the above reactions is respectively: Percentage strength: It expresses the amount of. Turn a beaker filled with oxygen upside down on top of a beaker that contains air to mix their contents, as shown in It destroys the colour of some organic compounds So, the match glowing strongly tells us that there is more oxygen in the lower beaker. It is invisible. State the utility or consequence of this ratio. Privacy Policy,
Many of the physical properties of hydrogen peroxide are similar to water. antiseptic and germicide for washing wounds, teeth and ears. With powerful oxidizing agents, H2O2 (d) State the method of collection of the oxygen gas giving reasons. Starling Bird: Species Identification & Overview | What is a Starling? Explore the preparation, properties, and structure of hydrogen peroxide. Incorrect answers will receive negative points. ferric salts . It does not change in quantity or in structure. All rights reserved.
Since beaker 2 is below beaker 1, oxygen, the heavier gas, will fall into beaker 2. Calculate the pH of a 6.7110?2 M NaOH solution. Chemistry, Q1) State the meaning of the terms 1 (a) Air 1 (b) Atmosphere. Copyright 2018-2023 BrainKart.com; All Rights Reserved.
The pollution due to oxides of sulphur gets enhanced due to the presence of : The correct IUPAC name of the following compound is: Identify the incorrect statement from the following.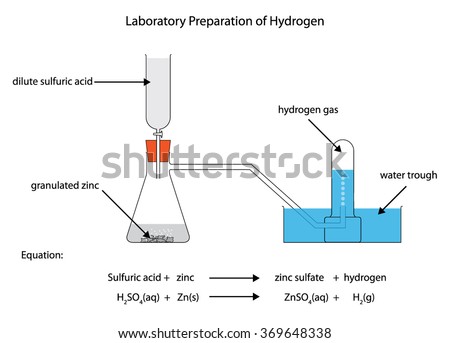 The other product is water. When barium peroxide is acidified and the excess water is removed by the process of evaporation under reduced pressure, we obtain hydrogen peroxide. If you were to do an Internet search for uses of hydrogen peroxide, you would come up with a large list. The most common method uses oxidation, which is when more carbon-oxygen bonds are formed, to oxidize hydroquinone, an aromatic compound derived from benzene, which acts as the source of hydrogens in the hydrogen peroxide, which acts as the hydrogen source. It reduces alkaline potassium ferricyanide. In this explainer, we will learn how to prepare oxygen gas and test some of its properties. Amira observed 4 different gases in glass cylinders. H2O2 is manufactured by followed by vacuum distillation. Oxygen makes up 21% of the volume of the atmosphere, as shown below. It's only in the presence of very strong oxidizing agent, such as magnesium oxide, that it can act as a reducing agent. Catalysts speed up a chemical reaction. It functions
Hydrogen peroxide decomposes back to water and oxygen when exposed to air and light. Look back at how we prepare oxygen from the decomposition of hydrogen peroxide. H2O2. 2. 2022 Quality Tutorials Pvt Ltd All rights reserved, Allied Solutions
The other product is water. When barium peroxide is acidified and the excess water is removed by the process of evaporation under reduced pressure, we obtain hydrogen peroxide. If you were to do an Internet search for uses of hydrogen peroxide, you would come up with a large list. The most common method uses oxidation, which is when more carbon-oxygen bonds are formed, to oxidize hydroquinone, an aromatic compound derived from benzene, which acts as the source of hydrogens in the hydrogen peroxide, which acts as the hydrogen source. It reduces alkaline potassium ferricyanide. In this explainer, we will learn how to prepare oxygen gas and test some of its properties. Amira observed 4 different gases in glass cylinders. H2O2 is manufactured by followed by vacuum distillation. Oxygen makes up 21% of the volume of the atmosphere, as shown below. It's only in the presence of very strong oxidizing agent, such as magnesium oxide, that it can act as a reducing agent. Catalysts speed up a chemical reaction. It functions
Hydrogen peroxide decomposes back to water and oxygen when exposed to air and light. Look back at how we prepare oxygen from the decomposition of hydrogen peroxide. H2O2. 2. 2022 Quality Tutorials Pvt Ltd All rights reserved, Allied Solutions
2HSO4- -- > < It acts as a bleaching agent for delicate material. Calculated quantity of Na2O2 is added in The only gas in the table that is not colored and has no odor is gas 4. Pure H2O2 Hydrogen peroxide was first prepared by L.J.Thenard, in 1813 by the action Test your knowledge on Hydrogen Peroxide Properties Preparation, NCERT Solutions Class 12 Business Studies, NCERT Solutions Class 12 Accountancy Part 1, NCERT Solutions Class 12 Accountancy Part 2, NCERT Solutions Class 11 Business Studies, NCERT Solutions for Class 10 Social Science, NCERT Solutions for Class 10 Maths Chapter 1, NCERT Solutions for Class 10 Maths Chapter 2, NCERT Solutions for Class 10 Maths Chapter 3, NCERT Solutions for Class 10 Maths Chapter 4, NCERT Solutions for Class 10 Maths Chapter 5, NCERT Solutions for Class 10 Maths Chapter 6, NCERT Solutions for Class 10 Maths Chapter 7, NCERT Solutions for Class 10 Maths Chapter 8, NCERT Solutions for Class 10 Maths Chapter 9, NCERT Solutions for Class 10 Maths Chapter 10, NCERT Solutions for Class 10 Maths Chapter 11, NCERT Solutions for Class 10 Maths Chapter 12, NCERT Solutions for Class 10 Maths Chapter 13, NCERT Solutions for Class 10 Maths Chapter 14, NCERT Solutions for Class 10 Maths Chapter 15, NCERT Solutions for Class 10 Science Chapter 1, NCERT Solutions for Class 10 Science Chapter 2, NCERT Solutions for Class 10 Science Chapter 3, NCERT Solutions for Class 10 Science Chapter 4, NCERT Solutions for Class 10 Science Chapter 5, NCERT Solutions for Class 10 Science Chapter 6, NCERT Solutions for Class 10 Science Chapter 7, NCERT Solutions for Class 10 Science Chapter 8, NCERT Solutions for Class 10 Science Chapter 9, NCERT Solutions for Class 10 Science Chapter 10, NCERT Solutions for Class 10 Science Chapter 11, NCERT Solutions for Class 10 Science Chapter 12, NCERT Solutions for Class 10 Science Chapter 13, NCERT Solutions for Class 10 Science Chapter 14, NCERT Solutions for Class 10 Science Chapter 15, NCERT Solutions for Class 10 Science Chapter 16, NCERT Solutions For Class 9 Social Science, NCERT Solutions For Class 9 Maths Chapter 1, NCERT Solutions For Class 9 Maths Chapter 2, NCERT Solutions For Class 9 Maths Chapter 3, NCERT Solutions For Class 9 Maths Chapter 4, NCERT Solutions For Class 9 Maths Chapter 5, NCERT Solutions For Class 9 Maths Chapter 6, NCERT Solutions For Class 9 Maths Chapter 7, NCERT Solutions For Class 9 Maths Chapter 8, NCERT Solutions For Class 9 Maths Chapter 9, NCERT Solutions For Class 9 Maths Chapter 10, NCERT Solutions For Class 9 Maths Chapter 11, NCERT Solutions For Class 9 Maths Chapter 12, NCERT Solutions For Class 9 Maths Chapter 13, NCERT Solutions For Class 9 Maths Chapter 14, NCERT Solutions For Class 9 Maths Chapter 15, NCERT Solutions for Class 9 Science Chapter 1, NCERT Solutions for Class 9 Science Chapter 2, NCERT Solutions for Class 9 Science Chapter 3, NCERT Solutions for Class 9 Science Chapter 4, NCERT Solutions for Class 9 Science Chapter 5, NCERT Solutions for Class 9 Science Chapter 6, NCERT Solutions for Class 9 Science Chapter 7, NCERT Solutions for Class 9 Science Chapter 8, NCERT Solutions for Class 9 Science Chapter 9, NCERT Solutions for Class 9 Science Chapter 10, NCERT Solutions for Class 9 Science Chapter 11, NCERT Solutions for Class 9 Science Chapter 12, NCERT Solutions for Class 9 Science Chapter 13, NCERT Solutions for Class 9 Science Chapter 14, NCERT Solutions for Class 9 Science Chapter 15, NCERT Solutions for Class 8 Social Science, NCERT Solutions for Class 7 Social Science, NCERT Solutions For Class 6 Social Science, CBSE Previous Year Question Papers Class 10, CBSE Previous Year Question Papers Class 12, JEE Main Chapter-wise Questions and Solutions, JEE Advanced Chapter-wise Questions and Solutions, Qualitative Analysis of Organic Compounds, JEE Main 2022 Question Papers with Answers. Q16) In the laboratory preparation of oxygen from hydrogen peroxide - answer the following: (a) Draw a neat labelled diagram for the method used in the above preparation. H2O2 is a powerful oxidizing agent. Hydrogen peroxide decomposes when exposed to sunlight, this process is catalyzed by traces of alkali metals. Enrolling in a course lets you earn progress by passing quizzes and exams. In the presence of oxygen this hydroquinone will oxidize into anthraquinone. Concentrated hydrogen peroxide is a very reactive oxygen species and is used as a propellant in rocketry. nitrogen, oxygen and carbon dioxide with reference Q6) Of the total volume of air - 4/5th is nitrogen. sodium peroxide.
When we prepare oxygen from the decomposition of hydrogen peroxide, we collect it over water. It is acidic in nature and PH is about 4.5.
1. To learn more about oxygen gas and its properties, we can prepare (make) it in the laboratory and perform some experiments. It's a nonplanar molecular with twisted symmetry and acts as a strong oxidizing agent. in the anhydrous state. While it's a great disinfecting agent for many things, doctors tend to not recommend using it on wounds anymore, because it slows the healing process.
 When hydrogen peroxide acts as an oxidizing agent, it gives up one of the oxygen atoms, leaving behind water as a byproduct. The bubbles happen when the hydrogen peroxide reacts with your living cells causing them to break down into water and oxygen. Oxygen can be prepared from the decomposition of hydrogen peroxide: Manganese dioxide is the catalyst in this reaction. The general equation for making hydrogen peroxide is to simply combine hydrogen with oxygen and hydrogen peroxide is formed. The melting point for hydrogen peroxide is -0.4 degrees Celsius, and the boiling point is 150 degrees Celsius. What is the other product of this reaction? column. It isn't shown on the image but this hydrogen is on a metal catalyst. Constant temperature T The, The IUPAC name of an element with atomic number 119 is. The structure of hydrogen peroxide is non-planar. Amongst the following which one will have maximum 'lone pair - lone pair' electron repulsions.
When hydrogen peroxide acts as an oxidizing agent, it gives up one of the oxygen atoms, leaving behind water as a byproduct. The bubbles happen when the hydrogen peroxide reacts with your living cells causing them to break down into water and oxygen. Oxygen can be prepared from the decomposition of hydrogen peroxide: Manganese dioxide is the catalyst in this reaction. The general equation for making hydrogen peroxide is to simply combine hydrogen with oxygen and hydrogen peroxide is formed. The melting point for hydrogen peroxide is -0.4 degrees Celsius, and the boiling point is 150 degrees Celsius. What is the other product of this reaction? column. It isn't shown on the image but this hydrogen is on a metal catalyst. Constant temperature T The, The IUPAC name of an element with atomic number 119 is. The structure of hydrogen peroxide is non-planar. Amongst the following which one will have maximum 'lone pair - lone pair' electron repulsions. The mass of oxygen compared to air can be investigated in a laboratory. Hydrogen peroxide acts as a reducing agent in this reaction. So, the correct answer is option C; manganese dioxide does not change in quantity or structure when used in the preparation The order of energy absorbed which is responsible for the color of complexes, The product formed from the following reaction sequence is. Hydrogen peroxide in both acidic and basic medium acts as an oxidizing as well as a reducing agent. Its like a teacher waved a magic wand and did the work for me. Hydrogen peroxide is a strong oxidizing agent. H2O2 is manufactured by followed by vacuum distillation. When hydrogen peroxide decomposes in the presence of manganese dioxide, oxygen is formed: The oxygen would escape in bubbles, making the hydrogen peroxide bubble and fizz. Reaction: H2O2 H2O + [O] Nascent oxygen. It is also used as a propellant in rockets.o Then, we see that the resulting anthraquinone can be exposed to hydrogen to reform the hydroquinone, and the reaction cycles back to the beginning. An error occurred trying to load this video. above on a water bath preferably under reduced pressure using fractionating We also can test if oxygen gas is soluble (dissolves) in water by placing a cylinder of gaseous oxygen upside down in water. Given below are two statements: one is labelled as Assertion (A) and the other is labelled as Reason (R). flashcard sets, {{courseNav.course.topics.length}} chapters | copyright 2003-2022 Study.com. It is used in the laboratory for detecting the presence of chromium, titanium, etc. (d) The method of collecting oxygen is downward displacement of water. This structure is not in a planar molecule but instead has a twisted symmetry. Molarity of hydrogen peroxide = 30.36/34 = 0.89 M. Also Read: What is Hydrogen Peroxide Used For. the image below. Laboratory preparation Earths atmosphere is The impurities like organic material or metallic We can then observe the level of the water in the cylinder: When we do this experiment, the water level rises only a small amount. 4. This tells us that gaseous oxygen scarcely dissolves When hydrogen peroxide is broken down in the presence of a black powder called manganese dioxide, new substances (products) 2. In this explainer, we have learned how to prepare oxygen gas and test some of its properties. This is an example of a decomposition reaction. sodium peroxide. Hydrogen peroxide can be an oxidizing agent or a reducing agent. Traces of H2O2 are found in atmosphere and How to Determine the Concentration of Hydrogen Peroxide?
hydrogenperoxideoxygengas+inthepresenceofmanganesedioxide. It should also be kept away from dust particles because dust can induce explosive decomposition of this compound. However, the boiling point is much higher than water, at 150 degrees Celsius. The strength of a sample of hydrogen peroxide | Urea Molecular Structure & Formula. volume of H2O2 gives on heating. 2HSO4(aq) [Electrolysis] HO3SOOSO3H(aq) [Hydrolysis] 2HSO4(aq)+2H+(aq)+H2O2(aq), 2HSO4 H2S2O8+ 2e Peroxodi Sulphuric Acid [Marshalls acid], H2S2O8+ H2O H2SO5+ H2SO4 Peroxomono Sulphuric Acid [Caros acid], Auto-Oxidation Method of Preparation of H2O2. feathers. Developed by Therithal info, Chennai. Fady and Lobna are talking about the oxygen produced when hydrogen peroxide decomposes. SAT Subject Test Physics: Tutoring Solution, Physical Science Curriculum Resource & Lesson Plans, ILTS Science - Environmental Science (112): Test Practice and Study Guide, SAT Subject Test Chemistry: Practice and Study Guide, ILTS Science - Chemistry (106): Test Practice and Study Guide, NY Regents Exam - Chemistry: Test Prep & Practice, High School Chemistry: Homework Help Resource, High School Physical Science: Tutoring Solution, NY Regents Exam - Chemistry: Tutoring Solution, NY Regents Exam - Physics: Help and Review, NY Regents Exam - Physics: Tutoring Solution, High School Physics: Homework Help Resource, Create an account to start this course today. Hydrogen peroxide has each oxygen connected to the other oxygen and to one of the hydrogen atoms. Pure H2O2 is unstable and decomposes on standing. and is used in bleaching delicate things like hair, wool, silk ivory and Oxidation is when more carbon-oxygen bonds are formed. materials like silk, wool, hair which will be destroyed by chlorine, are Find the emf of the cell in which the following reaction takes place at, The given graph is a representation of kinetics of a reaction. In this image, you can see that first we have hydroquinone.
Delicate If oxygen dissolves in the water, the water level rises, as the volume of oxygen gas decreases. Therefore, Fady is incorrect and only Lobna is correct. Oxygen can be prepared in a laboratory from a liquid called hydrogen peroxide. Put your understanding of this concept to test by answering a few MCQs. | Differentiated Instruction Resources, Customer Service Manager Skills & Training, High School US History Syllabus Resource & Lesson Plans, High School Trigonometry: Homework Help Resource, NMTA Elementary Education Subtest II (103): Practice & Study Guide, The Medieval Warm Period: Help and Review, Quiz & Worksheet - Lexically Ambiguous Sentences, Quiz & Worksheet - Ribosomes & Protein Synthesis, Quiz & Worksheet - David Wechsler on Intelligence, Quiz & Worksheet - Luddites During the Industrial Revolution, Motivational Tools for Students: Techniques & Examples, What Is Stomatitis? b) Write a balanced equation in which hydrogen peroxide reduces perman, Dr. Goodchem's favorite student made a new compound H_2O_2 Dr. student noted that the compound hat some of the same atoms as H_2O and is convinced that it would have the same physical and chemical pro, Select all of the true statements from the list below. Insert a burning match into the lower beaker. 3. (At anode), Concentration of Hydrogen peroxide can be found in glow sticks and rocket fuel, and can be used as an explosive or as a bleach for hair, paper, or flour. Get unlimited access to over 84,000 lessons. Collecting oxygen in this way would not be possible if oxygen dissolved easily in water. The bond angle between each oxygen and hydrogen is 102 degrees. The following diagram will clearly show what an open book structure means. Oxygen helps with burning. of dilute acid on barium peroxide. feathers. The following reaction will clarify this: BaO2.8H2O(s) + H2SO4(aq) BaSO4(s) + H2O2(aq) + 8H2O(l). Inorganic Chemistry Overview & Examples | What is Inorganic Chemistry? This would not be possible if oxygen dissolved in water. Ozone on reaction with hydrogen peroxide gives oxygen and water. It melts at 272.4 K and has a boiling point of 423 K (extrapolated). bleaching agent, powerful but harmless disinfectant and germicide.
 Q5) Compare the three main components of air i.e. are formed. So the melting point is very similar to that of water (which is 0 degrees Celsius), but the boiling point is much higher than the boiling point of water (which is 100 degrees Celsius). Therefore, H2O2 is stored in wax-lined glass or plastic containers and kept in dark. What experimental error usually occur in the reaction of hydrogen peroxide and bleach experiment? It acts as a bleaching agent and is also used as a disinfectant. in certain plants. To unlock this lesson you must be a Study.com Member. Try refreshing the page, or contact customer support. Kl is not a catalyst for the decomposition of hydrogen peroxide. On heating when water and oxygen are formed. as an electron acceptor. Laura has a Masters of Science in Food Science and Human Nutrition and has taught college Science. Class 7 It is in the air we breathe, the water we drink, and the food we eat. a mixture of gases that surround Earth. Hydrogen peroxide is prepared by the electrolysis of 30% ice-cold H2SO4. H2O2 + 2H+ + 2e-
water in the cylinder. Electronic Displacements in Covalent Bonds, Dobereiner's Law of Triads & Newlands' Law of Octaves, Oxidation of Alcohols | Reagents, Mechanism & Reaction, Common Ion Effect | Common Ion Chart, Effect on Solubility & Examples, De Broglie Equation Overview, Hypothesis & Examples | Wave Equation Formula, What is a Chemical Property? hydrogenperoxide solution. bleached with H. It destroys bacteria and hence it is used as an
of hydrogen peroxide.
Q5) Compare the three main components of air i.e. are formed. So the melting point is very similar to that of water (which is 0 degrees Celsius), but the boiling point is much higher than the boiling point of water (which is 100 degrees Celsius). Therefore, H2O2 is stored in wax-lined glass or plastic containers and kept in dark. What experimental error usually occur in the reaction of hydrogen peroxide and bleach experiment? It acts as a bleaching agent and is also used as a disinfectant. in certain plants. To unlock this lesson you must be a Study.com Member. Try refreshing the page, or contact customer support. Kl is not a catalyst for the decomposition of hydrogen peroxide. On heating when water and oxygen are formed. as an electron acceptor. Laura has a Masters of Science in Food Science and Human Nutrition and has taught college Science. Class 7 It is in the air we breathe, the water we drink, and the food we eat. a mixture of gases that surround Earth. Hydrogen peroxide is prepared by the electrolysis of 30% ice-cold H2SO4. H2O2 + 2H+ + 2e-
water in the cylinder. Electronic Displacements in Covalent Bonds, Dobereiner's Law of Triads & Newlands' Law of Octaves, Oxidation of Alcohols | Reagents, Mechanism & Reaction, Common Ion Effect | Common Ion Chart, Effect on Solubility & Examples, De Broglie Equation Overview, Hypothesis & Examples | Wave Equation Formula, What is a Chemical Property? hydrogenperoxide solution. bleached with H. It destroys bacteria and hence it is used as an
of hydrogen peroxide.  Gas 3 has odor, so it cannot be oxygen gas. is obtained by reacting BaO2 with an acid BaO2 + H2SO4 -- > Ba SO4 + H2O2. Manganese dioxide is a catalyst used in the preparation of oxygen from hydrogen peroxide. So, the answer is option D; after one minute, all of the oxygen will be in beaker 2 because it is heavier than air. It is also used as a propellant in rockets.o
- Formula, Production & Uses, AP EAMCET AM (Agriculture & Medical) Flashcards, High School Physics Curriculum Resource & Lesson Plans, Physics 101 Syllabus Resource & Lesson Plans, Middle School Physical Science Curriculum Resource & Lesson Plans, 7th Grade Physical Science: Enrichment Program, 6th Grade Physical Science: Enrichment Program, Physical Geology Syllabus Resource & Lesson Plans, NY Regents Exam - Earth Science: Tutoring Solution, ILTS Health Education (211): Test Practice and Study Guide, What Are Peroxide Antiseptics?
Gas 3 has odor, so it cannot be oxygen gas. is obtained by reacting BaO2 with an acid BaO2 + H2SO4 -- > Ba SO4 + H2O2. Manganese dioxide is a catalyst used in the preparation of oxygen from hydrogen peroxide. So, the answer is option D; after one minute, all of the oxygen will be in beaker 2 because it is heavier than air. It is also used as a propellant in rockets.o
- Formula, Production & Uses, AP EAMCET AM (Agriculture & Medical) Flashcards, High School Physics Curriculum Resource & Lesson Plans, Physics 101 Syllabus Resource & Lesson Plans, Middle School Physical Science Curriculum Resource & Lesson Plans, 7th Grade Physical Science: Enrichment Program, 6th Grade Physical Science: Enrichment Program, Physical Geology Syllabus Resource & Lesson Plans, NY Regents Exam - Earth Science: Tutoring Solution, ILTS Health Education (211): Test Practice and Study Guide, What Are Peroxide Antiseptics? H2O2 bleaches silk, wool, cotton, hair. It is a colourless liquid and is used in aqueous solution for safety reasons. In the pure state, hydrogen peroxide is almost colourless (very pale blue) liquid.
485 lessons Insert a burning match into the upper beaker. But it's still a disinfectant for many different surfaces and objects, including surgical instruments. because it is than air. | {{course.flashcardSetCount}} Moist silver oxide, acidified KMnO4, So, the correct answer is water; it is the other product of the reaction. By careful evaporation of the solution obtained What happens to manganese dioxide in this reaction? Oxygen does not dissolve in water, but it does above on a water bath preferably under reduced pressure using fractionating The Kjeldahl's method for the estimation of nitrogen can be used to estimate the amount of nitrogen in which one of the following compounds? We also know that a catalyst is a substance whose quantity and structure do not change during a chemical reaction. Click Start Quiz to begin! This fascinating liquid is called hydrogen peroxide. Peroxodisulphate is then hydrolyzed to get hydrogen peroxide. Please contact your portal admin. It may look empty, but in fact, it contains oxygen gas. {{courseNav.course.mDynamicIntFields.lessonCount}} lessons lessons in math, English, science, history, and more. By distillation under reduced pressure at Oxygen is scarcely soluble in water. Hydrogen peroxide is used for stain removal, as a cleanser and as a disinfectant. solution is expressed in terms of the volumes of oxygen at S.T.P that one Q2) Name the important scientists and their studies which led to the discovery of the components of Q3) Explain Lavoisers experiment which provided evidence to the discovery of components in air. in water. To calculate the molarity, Let us consider 10 Vol H2O2. When two gases are mixed, the heavier gas will fall to the bottom. volume of H, Due to its oxidizing property, it is a valuable It turns filter paper containing black stains of. 10 Vol H2O2 means 1 mL of H2O2 gives 10 mL of O2 at STP. It is used as an antiseptic for washing wounds. I would definitely recommend Study.com to my colleagues. We've never actually been able to boil hydrogen peroxide because, as we heat up hydrogen peroxide, it explodes and decomposes into water and oxygen. A catalyst is a substance whose quantity and structure do not change during a chemical reaction. The hydrogens, which were on the oxygen, now combine with the oxygen to form hydrogen peroxide. Having collected oxygen gas, we can investigate its properties. If the oxygen does not dissolve in the water, the water level will stay the same. Create your account, 57 chapters | (b) Catalyst used is manganese dioxide, it increases the speed of decomposition of H2O2. Which amongst the following is incorrect statement? the molar mass of H2O2 is 34.0147g/mol and the density of the solution is 1.000 g/ml. Hydrogen peroxide is the simplest kind of peroxide available (oxygen-oxygen single bond). By the action of dilute sulphuric acid on 30% solution of H2O2 is obtained by this process. -- > 2 H2O, It oxidizes ferrous salts int2Fe2+ + 2H+ + H2O2 --> 2 Fe3+ + 2H2O. The following reactions will give a clear picture: It bleaches by oxidation. H2O2 is a colourless, odourless, syrupy liquid Hydrogen Peroxide: Preparation, Properties & Structure, Heisenberg Uncertainty Principle: Definition & Equation, Valence Bond Theory of Coordination Compounds, Heterogeneous vs. Homogenous Catalysts | Overview, Differences & Examples, What is Urea? Plus, get practice tests, quizzes, and personalized coaching to help you Generally, it's prepared through oxidation of a hydroquinone. Additional Adjustments to Income for Taxes, Microscopic Procedures for Direct Examination of Parasite Specimens, Quiz & Worksheet - Features & Genres of Dance, Quiz & Worksheet - Death on the Nile Literary Elements, Flashcards - Real Estate Marketing Basics, Flashcards - Promotional Marketing in Real Estate, Science Worksheets | Printable Science Worksheets for Teachers, What is Differentiated Instruction? One of the products made when hydrogen peroxide breaks down is oxygen. It is used in the production of epoxides, inorganic chemicals like sodium perborate. Hydrogen peroxide is a bubbly, antimicrobial agent used as an environmentally friendly disinfectant. | Meaning, Types, & List of Examples, The Solvay Process: Process, Products & Environmental Issues, Nitric Acid Formula and Structure | Chemical Formula of Nitric Acid. Therefore, when oxygen and air are mixed, oxygen will fall to the bottom. (c) State the word equation for the reaction involving the above preparation of oxygen. 1.
Here. The physical properties of hydrogen peroxide are: The oxidation state of oxygen in hydrogen peroxide is -1. Catalysts speed up a It would sting but at the same time it was very intriguing because it would bubble and fizz. It's also used as a common alternative medicine treatment. Q4) Tabulate the various components of air, including the components with variable composition. The oxygen displaces the A hydroquinone is an aromatic compound derived from benzene, which acts as the source of hydrogens in the hydrogen peroxide. ferric salts . displace water. Heating samples to 100 degrees C f. Calculate the mass percent of the hydrogen peroxide in the old bottle. Let us have a look at the various methods of preparation for hydrogen peroxide. Traces of H2O2 are found in atmosphere and in certain plants. The experiment can be carried out in the laboratory using the equipment and steps shown below. formed. Look at this jar. - Definition, Types & Uses, Prokaryotic Algae Cells: Function, Definition & Features, Ascaris Parasitic Worms: Phylum & Classification, Ascaris Worms: Anatomy & Digestive Systems, Phylum Annelida Circulatory & Nervous Systems, Phylum Annelida Digestive & Respiratory Systems, Porifera Respiration & Respiratory System, Tapeworms in Humans: Symptoms & Treatment, TExES Science of Teaching Reading (293): Practice & Study Guide, Curriculum & Assessment in Music Education, Planned Value vs. Earned Value in Project Management, Difference Between There, Their & They're. column. Hydrogen peroxide is prepared in the laboratory by: Which one is not correct mathematical equation for Dalton's Law of partial pressure ? Gas 2 has color, so it cannot be oxygen gas. Identify the incorrect statement from the following. of oxygen from hydrogen peroxide. Since it does break down into two very harmless compounds, water and oxygen, it's seen as an environmentally friendly antimicrobial. The incorrect statement regarding chirality is : Which statement regarding polymers is not correct? We can investigate how heavy oxygen is compared to air using these steps: When we do this, we see that the match glows more strongly in the lower beaker. Study Material, Lecturing Notes, Assignment, Reference, Wiki description explanation, brief detail, 11th 12th std standard Class Organic Inorganic Physical Chemistry Higher secondary school College Notes : Hydrogen peroxide: Preparation, Properties, reactions, Uses |, Hydrogen peroxide: Preparation, Properties, reactions, Uses. The presence of oxygen makes a match glow brighter. One of Gas 1 has color and odor, so it cannot be oxygen gas. It destroys bacteria and hence it is used as an materials like silk, wool, hair which will be destroyed by chlorine, are (b) Name the catalyst used in the preparation and state its function. Terms and Conditions, Gadolinium has a low value of third ionisation enthalpy because of, Which one of the following is not formed when acetone reacts with 2-pentanone in the presence of dilute. Three particles have speeds of 2u10u and 11u Which class 11 chemistry JEE_Main, At very high pressure the compressibility factor of class 11 chemistry JEE_Main, Which of the following is not a gaseous fuel A CNG class 11 chemistry JEE_Main, An octahedral complex with molecular composition M5NH3ClSO4 class 11 chemistry JEE_Main, Amongst LiCl RbCl BeCl2 and MgCl2the compounds with class 11 chemistry JEE_Main, In which of the following pairs 1st is more stable class 11 chemistry JEE_Main, Epipetalous and syngenesious stamens occur in aSolanaceae class 11 biology CBSE, Differentiate between homogeneous and heterogeneous class 12 chemistry CBSE, Cl P Cl bond angles in PCl5 molecule is A 120 circ class 11 chemistry CBSE, Two vectors of equal magnitude have a resultant equal class 11 physics CBSE, A magnetic moment of 173BM will be shown by which one class 11 chemistry CBSE, Which of the following cannot be prepared by Sandmeyers class 11 chemistry CBSE, The maximum number of possible oxidation states of class 12 chemistry CBSE, What would happen to the life of a cell if there was class 11 biology CBSE, Name the members of the lanthanoid series which exhibit class 12 chemistry CBSE, CBSE Previous Year Question Paper for Class 10, CBSE Previous Year Question Paper for Class 12. I feel like its a lifeline. ions, may catalyse its explosive decomposition. Role of hydrogen peroxide in the above reactions is respectively: Percentage strength: It expresses the amount of. Turn a beaker filled with oxygen upside down on top of a beaker that contains air to mix their contents, as shown in It destroys the colour of some organic compounds So, the match glowing strongly tells us that there is more oxygen in the lower beaker. It is invisible. State the utility or consequence of this ratio. Privacy Policy,
Many of the physical properties of hydrogen peroxide are similar to water. antiseptic and germicide for washing wounds, teeth and ears. With powerful oxidizing agents, H2O2 (d) State the method of collection of the oxygen gas giving reasons. Starling Bird: Species Identification & Overview | What is a Starling? Explore the preparation, properties, and structure of hydrogen peroxide. Incorrect answers will receive negative points. ferric salts . It does not change in quantity or in structure. All rights reserved.
Since beaker 2 is below beaker 1, oxygen, the heavier gas, will fall into beaker 2. Calculate the pH of a 6.7110?2 M NaOH solution. Chemistry, Q1) State the meaning of the terms 1 (a) Air 1 (b) Atmosphere. Copyright 2018-2023 BrainKart.com; All Rights Reserved.
The pollution due to oxides of sulphur gets enhanced due to the presence of : The correct IUPAC name of the following compound is: Identify the incorrect statement from the following.
 The other product is water. When barium peroxide is acidified and the excess water is removed by the process of evaporation under reduced pressure, we obtain hydrogen peroxide. If you were to do an Internet search for uses of hydrogen peroxide, you would come up with a large list. The most common method uses oxidation, which is when more carbon-oxygen bonds are formed, to oxidize hydroquinone, an aromatic compound derived from benzene, which acts as the source of hydrogens in the hydrogen peroxide, which acts as the hydrogen source. It reduces alkaline potassium ferricyanide. In this explainer, we will learn how to prepare oxygen gas and test some of its properties. Amira observed 4 different gases in glass cylinders. H2O2 is manufactured by followed by vacuum distillation. Oxygen makes up 21% of the volume of the atmosphere, as shown below. It's only in the presence of very strong oxidizing agent, such as magnesium oxide, that it can act as a reducing agent. Catalysts speed up a chemical reaction. It functions
Hydrogen peroxide decomposes back to water and oxygen when exposed to air and light. Look back at how we prepare oxygen from the decomposition of hydrogen peroxide. H2O2. 2. 2022 Quality Tutorials Pvt Ltd All rights reserved, Allied Solutions
The other product is water. When barium peroxide is acidified and the excess water is removed by the process of evaporation under reduced pressure, we obtain hydrogen peroxide. If you were to do an Internet search for uses of hydrogen peroxide, you would come up with a large list. The most common method uses oxidation, which is when more carbon-oxygen bonds are formed, to oxidize hydroquinone, an aromatic compound derived from benzene, which acts as the source of hydrogens in the hydrogen peroxide, which acts as the hydrogen source. It reduces alkaline potassium ferricyanide. In this explainer, we will learn how to prepare oxygen gas and test some of its properties. Amira observed 4 different gases in glass cylinders. H2O2 is manufactured by followed by vacuum distillation. Oxygen makes up 21% of the volume of the atmosphere, as shown below. It's only in the presence of very strong oxidizing agent, such as magnesium oxide, that it can act as a reducing agent. Catalysts speed up a chemical reaction. It functions
Hydrogen peroxide decomposes back to water and oxygen when exposed to air and light. Look back at how we prepare oxygen from the decomposition of hydrogen peroxide. H2O2. 2. 2022 Quality Tutorials Pvt Ltd All rights reserved, Allied Solutions 2HSO4- -- > < It acts as a bleaching agent for delicate material. Calculated quantity of Na2O2 is added in The only gas in the table that is not colored and has no odor is gas 4. Pure H2O2 Hydrogen peroxide was first prepared by L.J.Thenard, in 1813 by the action Test your knowledge on Hydrogen Peroxide Properties Preparation, NCERT Solutions Class 12 Business Studies, NCERT Solutions Class 12 Accountancy Part 1, NCERT Solutions Class 12 Accountancy Part 2, NCERT Solutions Class 11 Business Studies, NCERT Solutions for Class 10 Social Science, NCERT Solutions for Class 10 Maths Chapter 1, NCERT Solutions for Class 10 Maths Chapter 2, NCERT Solutions for Class 10 Maths Chapter 3, NCERT Solutions for Class 10 Maths Chapter 4, NCERT Solutions for Class 10 Maths Chapter 5, NCERT Solutions for Class 10 Maths Chapter 6, NCERT Solutions for Class 10 Maths Chapter 7, NCERT Solutions for Class 10 Maths Chapter 8, NCERT Solutions for Class 10 Maths Chapter 9, NCERT Solutions for Class 10 Maths Chapter 10, NCERT Solutions for Class 10 Maths Chapter 11, NCERT Solutions for Class 10 Maths Chapter 12, NCERT Solutions for Class 10 Maths Chapter 13, NCERT Solutions for Class 10 Maths Chapter 14, NCERT Solutions for Class 10 Maths Chapter 15, NCERT Solutions for Class 10 Science Chapter 1, NCERT Solutions for Class 10 Science Chapter 2, NCERT Solutions for Class 10 Science Chapter 3, NCERT Solutions for Class 10 Science Chapter 4, NCERT Solutions for Class 10 Science Chapter 5, NCERT Solutions for Class 10 Science Chapter 6, NCERT Solutions for Class 10 Science Chapter 7, NCERT Solutions for Class 10 Science Chapter 8, NCERT Solutions for Class 10 Science Chapter 9, NCERT Solutions for Class 10 Science Chapter 10, NCERT Solutions for Class 10 Science Chapter 11, NCERT Solutions for Class 10 Science Chapter 12, NCERT Solutions for Class 10 Science Chapter 13, NCERT Solutions for Class 10 Science Chapter 14, NCERT Solutions for Class 10 Science Chapter 15, NCERT Solutions for Class 10 Science Chapter 16, NCERT Solutions For Class 9 Social Science, NCERT Solutions For Class 9 Maths Chapter 1, NCERT Solutions For Class 9 Maths Chapter 2, NCERT Solutions For Class 9 Maths Chapter 3, NCERT Solutions For Class 9 Maths Chapter 4, NCERT Solutions For Class 9 Maths Chapter 5, NCERT Solutions For Class 9 Maths Chapter 6, NCERT Solutions For Class 9 Maths Chapter 7, NCERT Solutions For Class 9 Maths Chapter 8, NCERT Solutions For Class 9 Maths Chapter 9, NCERT Solutions For Class 9 Maths Chapter 10, NCERT Solutions For Class 9 Maths Chapter 11, NCERT Solutions For Class 9 Maths Chapter 12, NCERT Solutions For Class 9 Maths Chapter 13, NCERT Solutions For Class 9 Maths Chapter 14, NCERT Solutions For Class 9 Maths Chapter 15, NCERT Solutions for Class 9 Science Chapter 1, NCERT Solutions for Class 9 Science Chapter 2, NCERT Solutions for Class 9 Science Chapter 3, NCERT Solutions for Class 9 Science Chapter 4, NCERT Solutions for Class 9 Science Chapter 5, NCERT Solutions for Class 9 Science Chapter 6, NCERT Solutions for Class 9 Science Chapter 7, NCERT Solutions for Class 9 Science Chapter 8, NCERT Solutions for Class 9 Science Chapter 9, NCERT Solutions for Class 9 Science Chapter 10, NCERT Solutions for Class 9 Science Chapter 11, NCERT Solutions for Class 9 Science Chapter 12, NCERT Solutions for Class 9 Science Chapter 13, NCERT Solutions for Class 9 Science Chapter 14, NCERT Solutions for Class 9 Science Chapter 15, NCERT Solutions for Class 8 Social Science, NCERT Solutions for Class 7 Social Science, NCERT Solutions For Class 6 Social Science, CBSE Previous Year Question Papers Class 10, CBSE Previous Year Question Papers Class 12, JEE Main Chapter-wise Questions and Solutions, JEE Advanced Chapter-wise Questions and Solutions, Qualitative Analysis of Organic Compounds, JEE Main 2022 Question Papers with Answers. Q16) In the laboratory preparation of oxygen from hydrogen peroxide - answer the following: (a) Draw a neat labelled diagram for the method used in the above preparation. H2O2 is a powerful oxidizing agent. Hydrogen peroxide decomposes when exposed to sunlight, this process is catalyzed by traces of alkali metals. Enrolling in a course lets you earn progress by passing quizzes and exams. In the presence of oxygen this hydroquinone will oxidize into anthraquinone. Concentrated hydrogen peroxide is a very reactive oxygen species and is used as a propellant in rocketry. nitrogen, oxygen and carbon dioxide with reference Q6) Of the total volume of air - 4/5th is nitrogen. sodium peroxide.
When we prepare oxygen from the decomposition of hydrogen peroxide, we collect it over water. It is acidic in nature and PH is about 4.5.
1. To learn more about oxygen gas and its properties, we can prepare (make) it in the laboratory and perform some experiments. It's a nonplanar molecular with twisted symmetry and acts as a strong oxidizing agent. in the anhydrous state. While it's a great disinfecting agent for many things, doctors tend to not recommend using it on wounds anymore, because it slows the healing process.